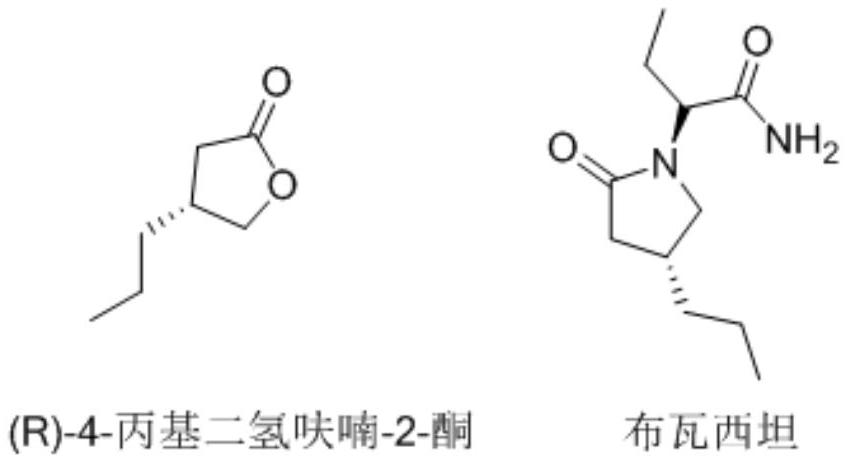
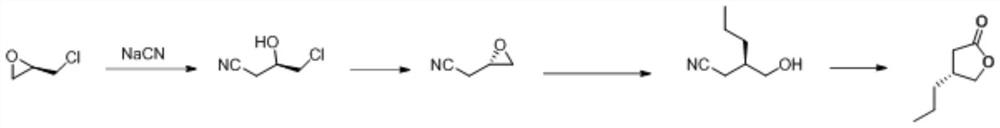
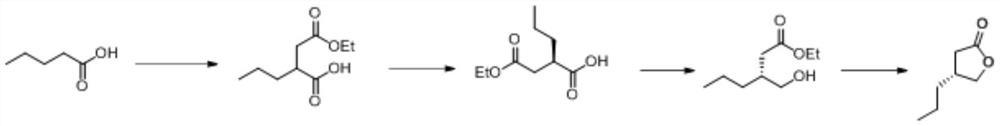
Synthesis method of (R)-4-propyldihydrofuran-2-ketone
A technology of propyl dihydrofuran and synthesis method, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of low product purity, high price, difficult production and amplification, etc., and achieve high optical purity, low cost, and novel route short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Synthesis of (1S,5R)-2-keto-3-oxabicyclohexane
[0024] Add ethanol (9L), sodium ethoxide (318.6g, 1.2eq.), stir at 0-5°C, add cycloisopropylidene malonate dropwise at 0-5°C (Menten's acid, 675g, 1.2eq. ), the dropwise addition was completed, and the reaction was kept at 0-5°C for 30 minutes; the temperature was naturally raised to 25°C (20-30°C), and R-epichlorohydrin (361g, 1.0eq.) was added dropwise. 20~30℃) to react for 10min; heat up to reflux reaction (78~83℃) to react for 8-12h until R-epichlorohydrin≤5%, drop to room temperature (25℃), dry under reduced pressure (jacket temperature ≤55℃), add 1.8L of water, 1.8L of ethyl acetate, stir, separate the liquids, extract the water phase with ethyl acetate (1.8L*2), combine the organic phases, add 720g of anhydrous sodium sulfate to dry for 30min, and filter with suction , the filter cake was rinsed with ethyl acetate, the filtrate was concentrated and dried under reduced pressure (jacket temperature ≤ 55°C), an...
Embodiment 2
[0028] (1) Synthesis of (1S,5R)-2-keto-3-oxabicyclohexane
[0029] Add ethanol (9L), sodium methoxide (1.2eq.), stir at 0-5°C, add cycloisopropylidene malonate (675g, 1.2eq.) dropwise at 0-5°C, after the addition is complete, Reaction at ~5°C for 30 minutes; naturally warm up to 25°C (20-30°C), add R-epichlorohydrin (361g, 1.0eq.) dropwise, after the addition is complete, react at 25°C (20-30°C) for 10 minutes ;Heat up to reflux reaction (78~83°C) for 8-12h, until R-epichlorohydrin≤5%, drop to room temperature (25°C), dry under reduced pressure (jacket temperature≤55°C), add water 1.8L, 1.8L ethyl acetate, stirring, liquid separation, extract the aqueous phase with ethyl acetate (1.8L*2), combine the organic phase, add 720g of anhydrous sodium sulfate to dry for 30min, filter with suction, and rinse the filter cake with ethyl acetate After washing, the filtrate was concentrated and dried under reduced pressure (jacket temperature≤55° C.), and the concentrated dry matter was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com