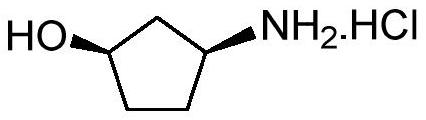
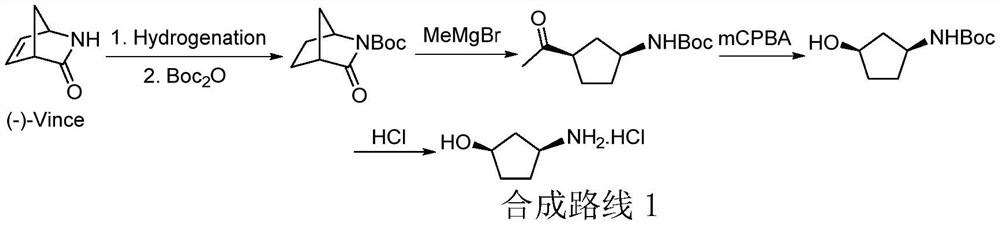
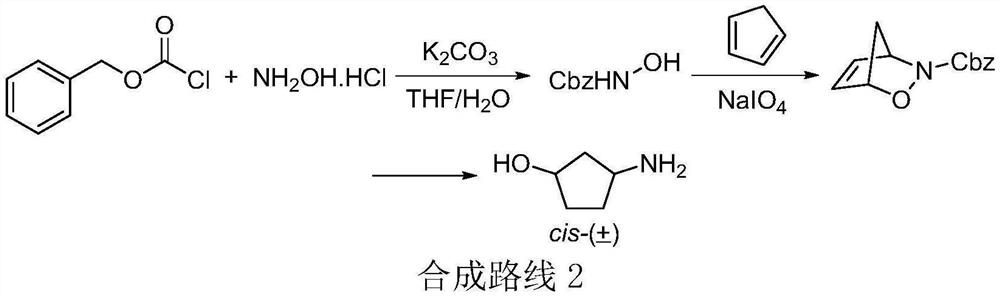
Method for preparing (1R, 3S)-3-aminocyclopentanol hydrochloride
A technology of aminocyclopentanol hydrochloride and carbamic acid, which is applied in the field of organic chemical synthesis, can solve the problems of difficult chiral control and high price, and achieve the effects of low cost, novel and short route, and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] (1) Synthesis of tert-butyl cis-2-oxa-3-azabicyclo[2.2.1]hept-5-ene-3-carboxylate
[0033] In a 3L reaction flask, tert-butyl carbonate-protected hydroxylamine (280 g, 2.1 mol) and 2-methyltetrahydrofuran (1 L) were sequentially added. Then start stirring, then add cyclopentadiene (208g, 3.2mol), 2-ethyl-2-oxazoline (17g, 0.20mol) and copper chloride (14g, 0.10mol) and copper chloride (14g, 0.10mol) successively in the reaction flask, and maintain The inner temperature of the reaction bottle was stirred for 10 minutes at 20-30°C. Then air (286 g, 2.5 mol) was slowly bubbled into the reaction flask, and the whole system was stirred and reacted under this condition for 12 hours. Afterwards, add water to dilute the system, and separate the liquids to obtain an organic phase, and then extract the aqueous phase with ethyl acetate. The combined organic phases were washed with saturated brine and dried, and the organic solvent was removed by rotary evaporation un...
Embodiment 2
[0050] Steps 1-2 and steps 4-6 in this implementation are the same as in Example 1.
[0051]
[0052] (3) Synthesis of cis-(+)-N-[4-hydroxyacetyl ester cyclopent-2-en-1-yl] tert-butyl carbamate
[0053]Intermediate (+ / -)-3 obtained in the third step was added to a 3L reaction flask, and then methylene chloride (830 mL), vinyl acetate (680 g, 8.0 mol, 5 equiv.) and Lipozyme40086 ( 22g). The whole reaction system was stirred and reacted at room temperature (25° C.) for 48 hours. Pad celite was filtered to remove the enzyme catalyst, and then the filtrate was concentrated by distillation under reduced pressure. The crude product was purified by silica gel column chromatography (elution machine: n-hexane / ethyl acetate mixed system) to obtain about 158 g of optically pure intermediate III, with a yield of 41% and an ee value >99%.
Embodiment 3
[0055] Steps 1-5 in this implementation are the same as in Example 1.
[0056]
[0057] (6) Synthesis of (1R,3S)-3-aminocyclopentanol hydrochloride
[0058] Add 250mL methanol to the dry reaction flask, then add acetyl chloride (70g, 0.89mol, 1.5equiv.) dropwise to the reaction flask under the condition of nitrogen protection, and keep the temperature of the system not exceeding 25 ℃, methanol solution of hydrogen chloride is generated in situ. After the methanol solution of hydrogen chloride was prepared, the intermediate was dissolved in 250 mL of methanol and added dropwise to the methanol solution of hydrogen chloride. After the dropwise addition, the whole system was reacted at room temperature (25° C.) for 12 hours. After the reaction is complete, distill and concentrate under reduced pressure to obtain a brown oily crude product, which is then recrystallized with isopropanol to obtain the target product (1R,3S)-3-amino-cyclopentanol hydrochloride as a white solid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com