A kind of preparation method of ticagrelor key intermediate
A technology of ticagrelor and chiral intermediates, which is applied in the field of medicine, can solve problems such as unsuitability for industrial production, difficulty in controlling chiral purity, and poor atom economy, so as to reduce labor and raw material costs, and avoid material loss and cost Effects of pressure, shortening of reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
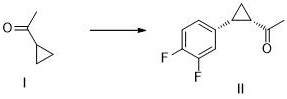
[0040] Formula I compound obtains formula II compound through coupling reaction
[0041]
[0042] Cyclopropylmethyl ketone (1.68g, 20mmol), palladium acetate (0.22g, 1mmol), L-valine (0.23g, 2mmol), 2-hydroxy-5-nitropyridine (0.28g, 2mmol), Add 3,4-difluoroiodobenzene (2.40g, 10mmol), trifluoroacetic acid (1.71g, 15mmol), and silver phosphate (2.76g, 10mmol) into 25mL of isopropanol, heat to reflux, keep the reaction overnight, and detect by TLC The starting material was completely converted. An appropriate amount of silica gel was added, concentrated to dryness at 45°C under reduced pressure, and 1.70 g of the compound of formula II was obtained by column chromatography. The purity was 98.5% by HPLC, the yield was 85.0%, and the ee value was >99%. 1 H NMR (500MHz, CDCl 3 )δ7.03(ddd, J=8.9,7.5,5.6Hz,1H),6.99-6.92(m,1H),6.89(dddd,J=7.6,5.7,2.0,1.0Hz,1H),2.60-2.51( m,1H),2.36(q,J=7.0Hz,1H),2.19(s,3H),1.71(td,J=7.0,5.0Hz,1H),1.39(td,J=6.9,4.9Hz,1H ).
Embodiment 2
[0044]Cyclopropylmethyl ketone (1.68g, 20mmol), tetrakis(triphenylphosphine) palladium (0.23g, 0.2mmol), L-proline (0.12g, 1mmol), 2-hydroxy-5-trifluoroform Pyridine (0.06g, 0.4mmol), 3,4-difluoroiodobenzene (3.60g, 15mmol), trifluoroacetic acid (4.56g, 40mmol), silver acetate (0.47g, 4mmol) were added into 30mL acetonitrile and heated to Reflux, keep the reaction overnight, and TLC detects that the starting material is completely converted. An appropriate amount of silica gel was added, concentrated to dryness at 45°C under reduced pressure, and 1.67 g of the compound of formula II was obtained by column chromatography. The purity was 97.6% by HPLC, the yield was 83.0%, and the ee value was >99%.
Embodiment 3
[0046] The compound of formula II is prepared by a one-pot method of configuration inversion and haloform reaction to compound of formula III
[0047]
[0048] Dissolve the compound of formula II (1.96g, 10mmol) in 10mL of 1,4-dioxane, add aqueous sodium hydroxide solution (5mL, 80mmol), heat up to 40°C, keep stirring overnight, and TLC monitors that the configuration is completely inverted. Cool down to 0-5°C, slowly add bromine (6.39g, 40mmol) dropwise, and control the temperature not to exceed 5°C; after dropping, keep warm for 6h, add sodium thiosulfate (3.16g, 20mmol), stir at room temperature for 30min, and react The solution was adjusted to pH 3-4 with 2N hydrochloric acid. Extracted with 10mL*3 ethyl acetate, layered, washed with 10mL*2 water, the organic layer was dried with anhydrous sodium sulfate, filtered, and an appropriate amount of silica gel was added to the filtrate , concentrated to dryness at 45°C under reduced pressure, and obtained 1.94 g of the compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com