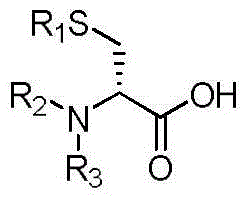
(S)-4-amino-5-mercaptopentanoic acid preparation method
A technology of mercaptovaleric acid and amino, which is applied in the field of medicine, can solve cumbersome problems, and achieve the effects of simple washing operation, high yield and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of GluSH
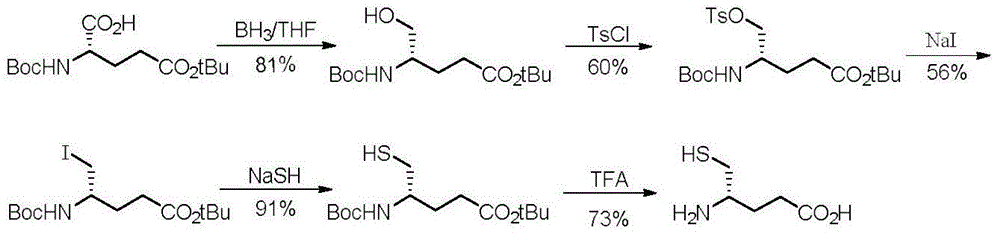
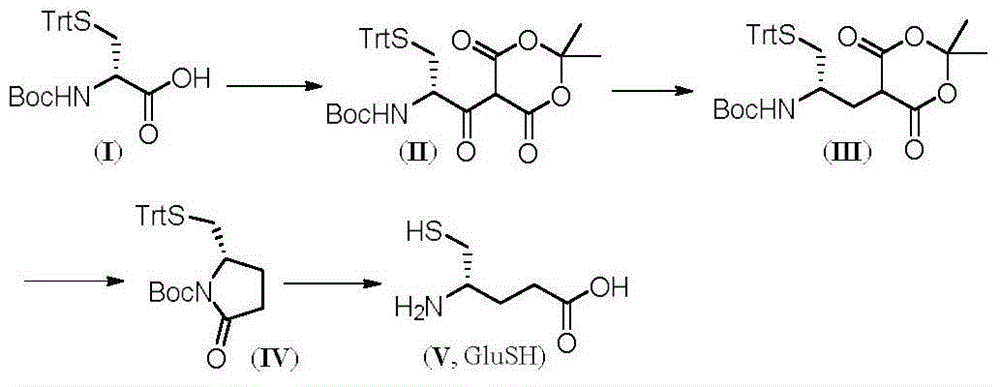
[0026] Through the following reaction route:
[0027]
[0028] Follow the steps below:
[0029] In the first step, D-cysteine with protected sulfhydryl and amino groups is condensed with Michaelis acid to generate β-ketoester:
[0030] Boc-S-trityl-D-cysteine (5.00 g) and Michaelis acid (1.71 g) were dissolved in 100 mL of dry dichloromethane in turn, and 4-dimethylaminopyridine ( 1.98g), after stirring in an ice bath for 10min, dicyclohexylcarbodiimide (2.45g) was added, and after stirring for 8h under ice bath conditions, liquid mass spectrometry (LC-MS) and thin layer chromatography (TLC) showed that the reaction complete, add saturated ammonium chloride to quench the reaction, and wash with 1M hydrochloric acid, saturated sodium bicarbonate and saturated sodium chloride successively, dry over anhydrous sodium sulfate, concentrate under reduced pressure to obtain a light brown powdery solid, wash twice with ether Finally, 5....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com