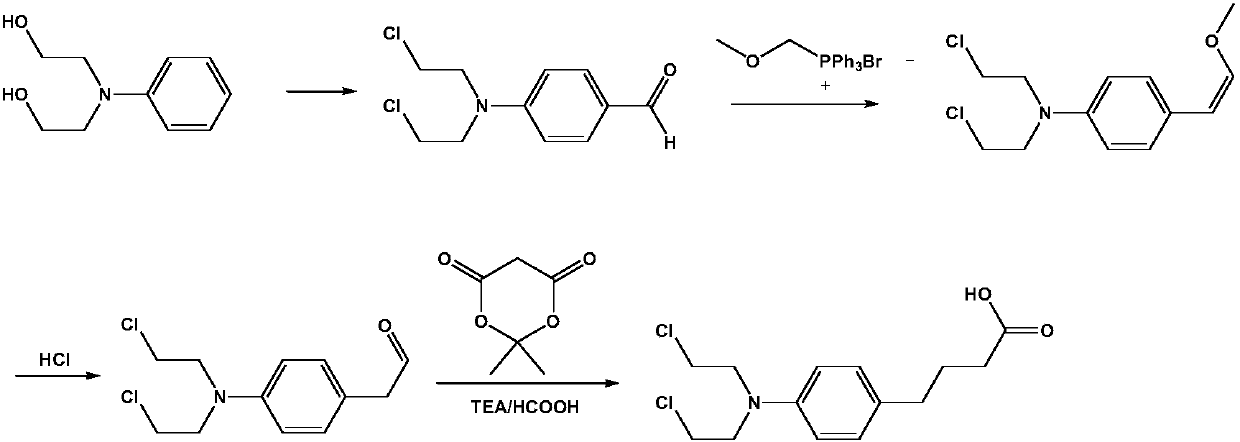
Preparation method of anti-tumor medicine chlorambucil
A technology for chlorambucil and an antitumor drug, which is applied in the field of compound preparation, can solve the problems of harsh equipment conditions, high production risk, difficult purification and the like, and achieves the effects of high cost, low production cost, and easy purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] The invention provides a kind of preparation method of antineoplastic drug chlorambucil, the method comprises the following steps:
[0024] (1) Under the condition of ice-salt bath at 0~5℃, according to the mass ratio of N,N-dimethylformamide: phosphorus oxychloride=2~5:1, drop into N,N-dimethylformamide Add phosphorus oxychloride, during the dropwise addition of phosphorus oxychloride, control the system temperature T≤45°C, stir for 0.5~1.0h after the dropwise addition is completed within 1~2h; then add dropwise concentration of 40~45wt%N,N- The dimethylformamide solution of dihydroxyethylaniline, after the dropwise addition within 2 to 3 hours, react at room temperature for 0.4 to 0.5 hours, raise the temperature to 85 to 100°C, and react for 5 to 6 hours; after the reaction is completed, slowly pour the reaction system into Terminate the reaction in ice water, add sodium bicarbonate solution dropwise to adjust the pH to 8-9, filter with suction and wash with water to...
Embodiment 1
[0035] The preparation method of antitumor drug chlorambucil, the steps are as follows:
[0036] (1) Add 210g of phosphorus oxychloride dropwise to 1000g of N,N-dimethylformamide under the condition of ice-salt bath at 2°C. During the dropping process, control the temperature T at about 40°C. After 1.3 hours of dropwise addition , stirred for 0.6h. Then a mixed solution of 80g N,N-dihydroxyethylaniline and 100g dimethylformamide was added dropwise at room temperature 25°C, the dropwise addition was completed after 2h and the reaction was maintained at room temperature for 0.5h, then the temperature was raised to 90°C for 5h. After the reaction is complete, slowly pour the system into 3000g of ice water to terminate the reaction, add dropwise 1mol / L sodium bicarbonate solution to adjust the base to pH=8, filter the obtained solid with suction, wash with water to obtain a white solid, dry it to obtain 120g, add 960g of acetic acid Ethyl ester was recrystallized, filtered and dr...
Embodiment 2
[0041] The preparation method of antitumor drug chlorambucil, the steps are as follows:
[0042] (1) Under the condition of ice-salt bath at 5°C, add 1050g of phosphorus oxychloride dropwise to 2500g of N,N-dimethylformamide. During the dropping process, control the temperature T at about 35°C. After 2 hours of dropping, Stir for 1.0 h. Then a mixed solution of 200g N,N-dihydroxyethylaniline and 250g dimethylformamide was added dropwise at room temperature 23°C, the dropwise addition was completed in 3h and the reaction was maintained at room temperature for 0.5h, then the temperature was raised to 90°C for 6h. After the reaction is complete, slowly pour the system into 3000g of ice water to terminate the reaction, add dropwise 1mol / L sodium bicarbonate solution to adjust the base to pH = 9, filter the obtained solid with suction, wash with water to obtain a white solid, dry it to obtain 303g, add 2200g of formic acid Ethyl ester was recrystallized, and dried by suction filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com