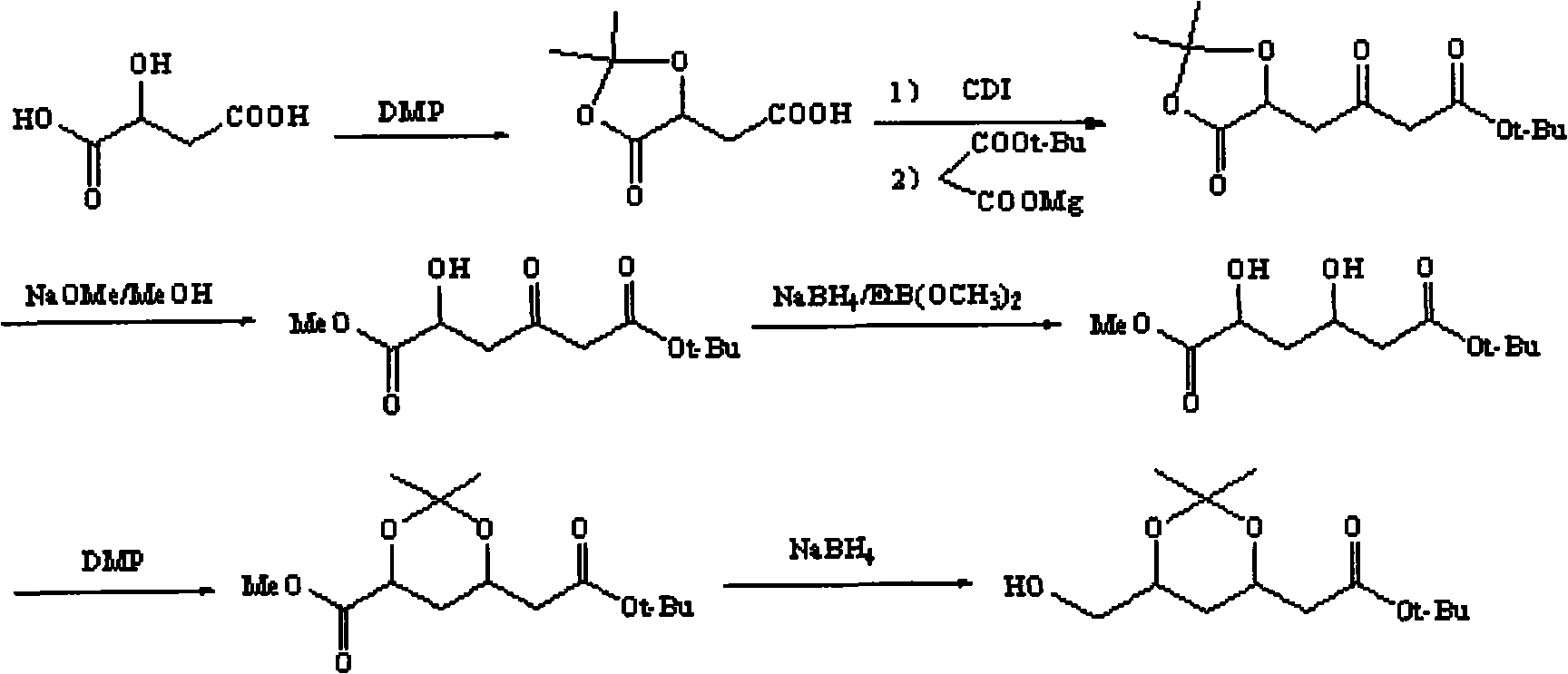
Method for preparing (3R, 5S)-3, 5-O-isopropylidene-3, 5, 6-trihydroxy caproic acid hexylic acid derivative
A technology of trihydroxycaproic acid and isopropylidene, applied in the field of preparation-3, can solve the problems of anhydrous operation price, unsuitable for scale-up production, impurity influence reaction, etc., achieves high quality and yield, is convenient for industrialized implementation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
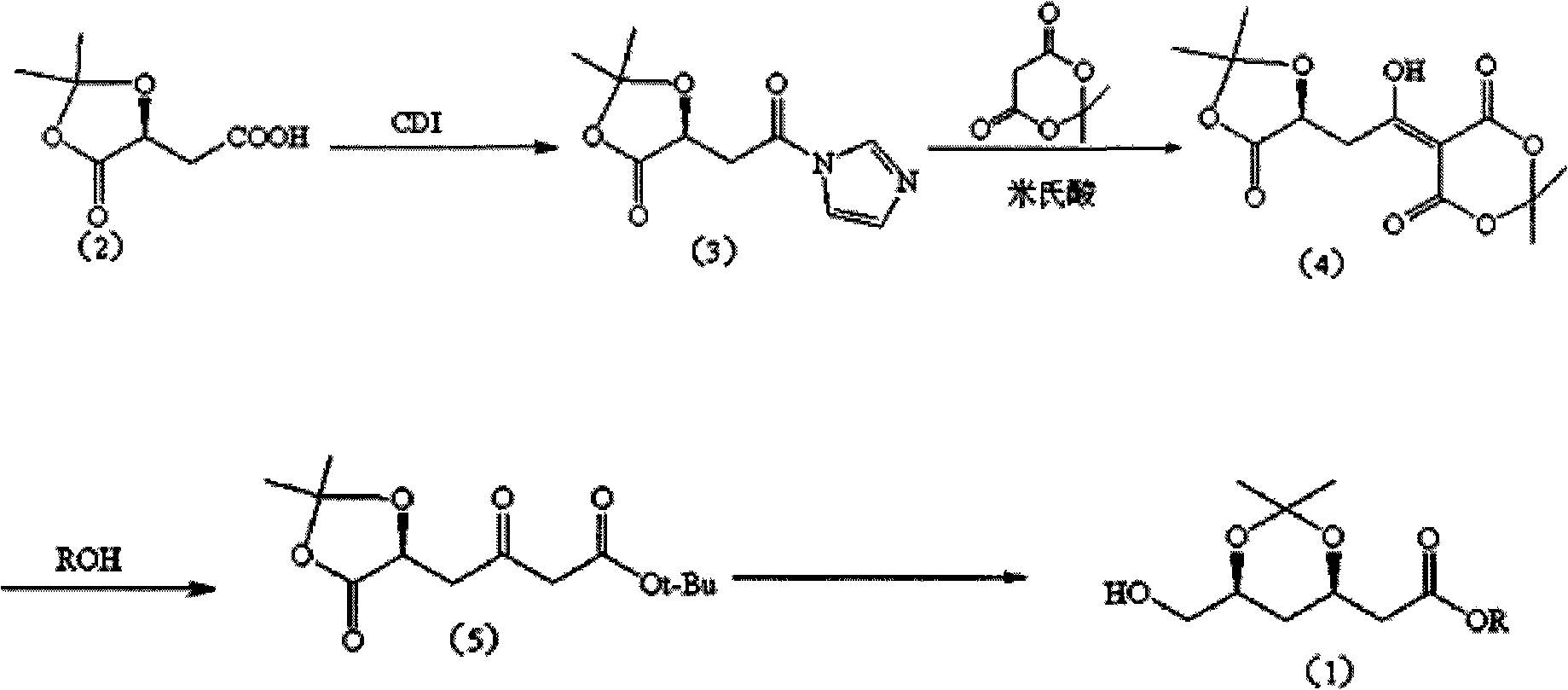
[0033] 1. Preparation of (S)-2,2-dimethyl-5-oxo-1,3-dioxa-4-acetylimidazole (3):
[0034] 19.5g of compound (2) was dissolved in 150ml of dichloromethane, 18.2g of carbonyldiimidazole (CDI) was added, and after reacting at 25°C for 2h, it was concentrated to obtain 25g of yellow solid; then recrystallized to obtain 21.3g of white flaky solid ( S)-2,2-Dimethyl-5-oxo-1,3-dioxa-4-acetylimidazole (3). mp: 126.9-128.3°C.
[0035] MS: 225(M+1)
[0036] 1 H-NMR (CDCl 3 ): 8.18(s, 1H), 7.48(s, 1H), 7.13(s, 1H), 4.91(m, 1H), 3.28-3.54(dd, 2H), 1.57-1.65(d, 6H)
[0037] 2. (S)-2-(4'-oxo-3', 4'-O-isopropylidene-3', 4'-dihydroxy-1'-hydroxyl-ene)-malonate The preparation of isopropyl ester (4):
[0038] 21.3g of compound (3), 13.6g of Michaelis acid and 6.5g of imidazole were dissolved in 250ml of dichloromethane, stirred at 25°C for 15h, concentrated to obtain 24.2g of crude yellow oil.
[0039] Take part of the crude product column chromatography to obtain light yellow oil (S)-2-(...
Embodiment 2
[0048] 1. Preparation of (S)-2,2-dimethyl-5-oxo-1,3-dioxa-4-acetylimidazole (3):
[0049] Dissolve 20g of compound (2) in 200ml of acetonitrile, add 18.6g of carbonyldiimidazole (CDI), react at 70°C for 1 hour, concentrate to obtain 25g of yellow solid; then recrystallize to obtain 15.4g of white flaky solid (S)- 2,2-Dimethyl-5-oxo-1,3-dioxa-4-acetylimidazole (3). mp: 127-128.3°C.
[0050] MS: 225(M+1)
[0051] 1 H-NMR (CDCl 3 ): 8.18(s, 1H), 7.48(s, 1H), 7.13(s, 1H), 4.91(m, 1H), 3.28-3.54(dd, 2H), 1.57-1.65(d, 6H)
[0052] 2. (S)-2-(4'-oxo-3', 4'-O-isopropylidene-3', 4'-dihydroxy-1'-hydroxyl-ene)-malonate The preparation of isopropyl ester (4):
[0053] 15.4g of compound (3), 19.8g of Michaelis acid and 11.1ml of pyridine were dissolved in 200ml of tetrahydrofuran, stirred at 0°C for 48h, concentrated to obtain 10.3g of crude yellow oil.
[0054] Take part of the crude product column chromatography to obtain light yellow oil (S)-2-(4'-oxo-3',4'-O-isopropylidene-3',4'-di...
Embodiment 3
[0063] 1. Preparation of (S)-2,2-dimethyl-5-oxo-1,3-dioxa-4-acetylimidazole (3):
[0064] Dissolve 20g of compound (2) in 200ml of tetrahydrofuran, add 55.8g of carbonyldiimidazole (CDI), react at 10°C for 5h, concentrate to obtain 28g of yellow solid; then recrystallize to obtain 18g of white flaky solid (S)-2, 2-Dimethyl-5-oxo-1,3-dioxa-4-acetylimidazole (3). mp: 126.5-128.4°C.
[0065] MS: 225(M+1)
[0066] 1 H-NMR (CDCl 3 ): 8.18(s, 1H), 7.48(s, 1H), 7.13(s, 1H), 4.91(m, 1H), 3.28-3.54(dd, 2H), 1.57-1.65(d, 6H)
[0067] 2. (S)-2-(4'-oxo-3', 4'-O-isopropylidene-3', 4'-dihydroxy-1'-hydroxyl-ene)-malonate The preparation of isopropyl ester (4):
[0068] 18g of compound (3), 34.7g of Michaelis acid and 24.3g of triethylamine were dissolved in 250ml of acetonitrile, stirred at 80°C for 1h, concentrated to obtain 7.2g of crude yellow oil.
[0069] Take part of the crude product column chromatography to obtain light yellow oil (S)-2-(4'-oxo-3',4'-O-isopropylidene-3',4'-dih...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com