Method for synthesizing 4-hydroxyl coumarin and derivant thereof
A technology for hydroxycoumarin and derivatives, which is applied in the field of synthesizing 4-hydroxycoumarin and derivatives thereof, can solve the problems of high price of o-hydroxyacetophenone, increased industrial production danger, strict reaction conditions, etc. It is beneficial to large-scale industrial production, the reaction process is less difficult, and the equipment investment is less.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
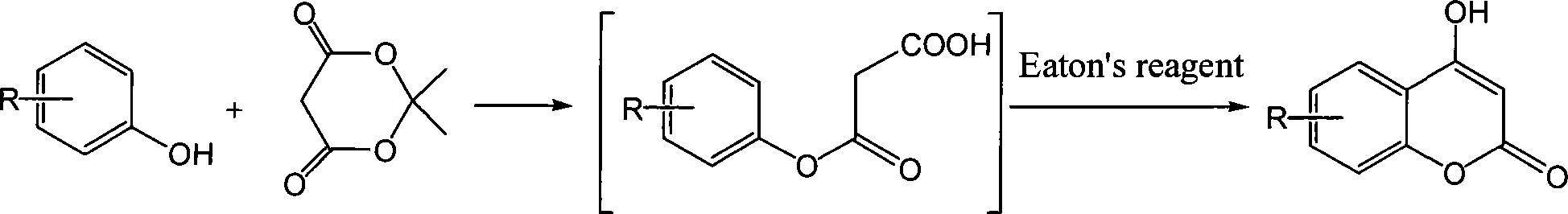
[0043] Using phenol or its derivatives and Meldrum's acid as raw materials, it is monitored by thin layer method (TLC). After the reaction is complete, the by-product acetone in the middle is removed under reduced pressure, and the acetone can be recycled to further realize clean production; The product does not need to be separated, and it is directly dehydrated with Ito's reagent (Eaton's Reagent) for intramolecular dehydration and ring closure reaction. Finally, water is added to precipitate the precipitate, filtered, and recrystallized to obtain the final product, namely 4-hydroxycoumarin and its derivatives. The reaction formula is as follows:
[0044]
[0045] in:
[0046] The substituent R can be one or more electron-donating groups or electron-withdrawing groups, and the substituent R electron-donating groups can be methyl, ethyl, tert-butyl and other alkyl groups, methoxy groups, ethyl groups, etc. An alkoxy group such as oxy group can also be a benzene ring; an el...
Embodiment 2
[0049] Phenol (1.0 mmol) and Michaelis acid (1.0 mmol) were added into a 10 mL round bottom flask and mixed, heated and stirred at 100° C. for 3 hours, and cooled to room temperature. vacuum filtration to remove residual acetone; and recycle acetone. Add 3mL Ito reagent, heat and stir at 70°C for 5 hours, cool to room temperature, add 20mL water while stirring, then cool to room temperature, filter to obtain crude product, recrystallize with 3-5mL ethanol and 1-3mL water to obtain 4-Hydroxycoumarin, yellow solid, yield 60.5%, melting point: 213.0-214.3°C.
Embodiment 3
[0051] Add p-tert-butylphenol (1.0 mmol) and Michaelis acid (1.0 mmol) into a 10 mL round-bottomed flask, mix, heat and stir at 100° C. for 3 hours, and cool to room temperature. vacuum filtration to remove residual acetone; and recycle acetone. Add 3 mL of Ito reagent, heat and stir at 70°C for 1 hour, cool to room temperature, add 20 mL of water while stirring, then cool to room temperature, filter to obtain a crude product, recrystallize with 3-5 mL of ethanol and 1-3 mL of water to obtain 4 -Hydroxy-6-tert-butylcoumarin, yellow powder, yield 82.6%, melting point: 216.0-218.2°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com