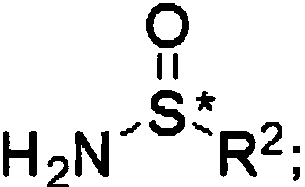Chiral synthesis method for chiral beta-amino acid and synthesis method for medicinal intermediate
A chiral synthesis and amino acid technology, which is applied in the preparation of carbamic acid derivatives, organic chemical methods, chemical instruments and methods, etc., can solve the problems of inconvenient large-scale preparation and storage, high cost of oxidants, and inconvenience of sitagliptin intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
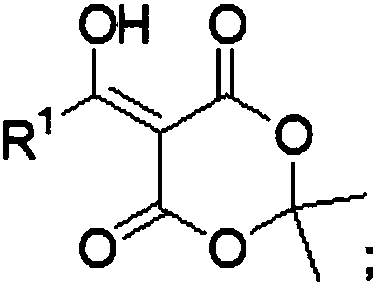
[0062] 5-[2-(2,4,5-trifluorophenyl)-1-hydroxyethylidene]-2,2-dimethyl-1,3-dioxane-4,6-dione preparation
[0063]
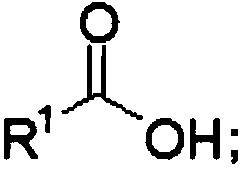
[0064] Under ice bath conditions, into a 2L three-neck reaction flask equipped with 800mL acetonitrile, add 2,4,5-trifluorophenylacetic acid (285.2g, 1.5mol, 1eq), pivaloyl chloride (199.0g, 1.65mol, 1.1eq ), DMAP (1.8g, 0.015mol, 0.01eq), DIPEA (387.6g, 3.0mol, 2.0eq), stirred at room temperature for 45min, added Michaelis acid (237.8, 1.65mol, 1.1eq) and raised the temperature to 45°C to react overnight. TLC detects the progress of the reaction. After the reaction was completed, cool to room temperature, slowly add 1M hydrochloric acid, and a solid was formed. After the obtained solid was washed with water, it was recrystallized from acetonitrile-water to obtain a white solid with a yield of 85%. The NMR results are the same as those reported by F.Xu, J.D.Armstrong, G.X.Zhou, B.Simmons, D.Hughes, Z.Geand E.J.J.Grabowski, J.Am.Chem.Soc., 2004, 126, 13002–13...
Embodiment 2
[0066] (R)-N-[1-(2,2-Dimethyl-4,6-dione-1,3-dioxane-5-ylidene)-2-(2,4,5-tri Preparation of fluorophenyl)ethyl]-2-methylpropane-2-sulfinamide
[0067]
[0068] Under the condition of stirring at room temperature, add the compound (316.2g, 1.0mol, 1eq) obtained in Example 1 into a 2L three-neck reaction flask equipped with 800mL of acetonitrile, and after it is completely dissolved, add (R)- tert-Butylsulfinamide (133.3g, 1.1mol, 1.1eq), after the addition, the system was slowly warmed up to room temperature, stirred overnight at 65°C, and the reaction progress was detected by TLC. After the reaction was completed, most of the solvent was removed, water was added, and Extract with methyl chloride, combine the organic phases, dry over anhydrous sodium sulfate, spin off the solvent, and crystallize from the solution of methanol and dichloromethane to obtain a white solid with a yield of 81%.
Embodiment 3
[0070] (R)-N-[(R)-1-(2,2-Dimethyl-4,6-dione-1,3-dioxane-5-ylidene)-2-(2,4 , Preparation of 5-trifluorophenyl)ethyl]-2-methylpropane-2-sulfinamide
[0071]
[0072] Under the condition of stirring at room temperature, in the 2L three-neck reaction flask equipped with 800mL ethanol, add the compound obtained in Example 2 (209.7g, 0.5mol, 1eq), after it is completely dissolved, slowly add sodium borohydride (56.7 g, 1.5mol, 3eq), after the addition, the system was slowly warmed up to room temperature, after stirring for 3 hours, stirred overnight at 65°C, and the reaction progress was detected by TLC. EA was extracted, the organic phases were combined, dried over anhydrous sodium sulfate, the organic solvent was spun off, and dichloromethane petroleum ether was crystallized to obtain the product (82%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com