Synthetic method of oat alkaloids
A technology of oat alkaloids and synthesis methods, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve problems such as harsh reaction conditions and complicated preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
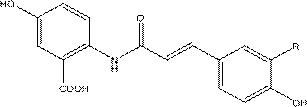
[0046] Synthesis of 2-(2-carboxyacetamido)-5-hydroxybenzoic acid
[0047] Weigh 15.3 g (0.1 mol) and 15.1 g (0.105 mol) of 2-amino-5-hydroxybenzoic acid and 15.1 g (0.105 mol) Michaelis acid, add them to a 500 mL round-bottomed flask, fully disperse them evenly with 300 mL of anhydrous toluene, and heat to reflux for reaction After 6-8 hours, cool to room temperature, remove the toluene by suction filtration to obtain an off-white solid, wash twice with toluene, and air-dry the obtained off-white solid in a fume hood, wait for the toluene to volatilize, and recrystallize the product after drying. The synthesized primary product of 2-(2-carboxyacetamido)-5-hydroxybenzoic acid was stirred evenly with ice water, and sodium hydroxide solution was slowly added dropwise until all the products were dissolved and the solution was light purple, and the stirring was continued for 30 min. Then add cold hydrochloric acid solution (6 mol / L), and add ice cubes to the reaction solution to co...
Embodiment 2
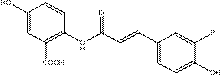
[0053] Synthesis of avenous alkaloid B,
[0054] Weigh 2.39 g (10 mmol) of 2-(2-carboxyacetamido)-5-hydroxybenzoic acid and 1.28 g (10.5 mmol) of 3-methoxy-4-hydroxybenzaldehyde and add them to a 100 mL double-necked circular In the bottom flask, fully dissolve with 20 mL of pyridine, then add the catalyst β-alanine (0.2 mmol), and heat to 90 o C, cooled to room temperature after 24 hours of reaction. After the reaction was completed, the reaction solution was poured into a 500 mL beaker, ice cubes were added, and the solution was cooled to 0 o C, then slowly add dilute hydrochloric acid solution, add ice cubes in time to control the temperature at 0 o About C, adjust the pH value to 2~3, precipitate a large amount of light yellow solid, stand still, filter with suction, wash the product with a large amount of deionized water, and recrystallize the product oat alkaloid B with hot acetone and water, and wash the initial product with acetone Heat and stir to dissolve, then ...
Embodiment 3
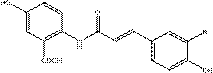
[0057] Synthesis of avenous alkaloid C,
[0058] Weigh 2.39 g (10 mmol) of 2-(2-carboxyacetamido)-5-hydroxybenzoic acid and 1.28 g (10.5 mmol) of 3,4-dihydroxybenzaldehyde into a 100 mL double-neck round bottom flask , fully dissolved with 20 mL of pyridine, then added catalyst β-alanine (0.2 mmol), heated to 90 o C, cooled to room temperature after 24 hours of reaction. After the reaction was completed, the reaction solution was poured into a 500 mL beaker, ice cubes were added, and the solution was cooled to 0 o C, then slowly add dilute hydrochloric acid solution, add ice cubes in time to control the temperature at 0 o About C, adjust the pH value to 2~3, precipitate a large amount of yellow-green solid, stand still, filter with suction, the product is washed with a large amount of deionized water, the product oat alkaloid C is further recrystallized with hot acetone and water, and the initial product is washed with acetone Heat and stir to dissolve, then slowly add de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com