Industrial preparation method of acetyl tetrahydrofuran with high optical purity
A technology of acetyltetrahydrofuran and optical purity, which is applied in the industrialized preparation field of acetyltetrahydrofuran with high optical purity, can solve the problems of difficult product purification, complicated operation and high cost, and achieves the effects of stable quality, low preparation cost and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
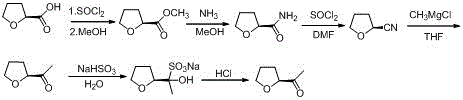
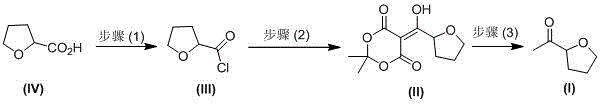
[0035] A kind of industrial preparation method of high optical purity S-acetyl tetrahydrofuran, comprises the following steps:
[0036] (1) S-tetrahydrofuroyl chloride ( Ⅲ ) preparation
[0037] Add 100Kg of S-tetrahydrofurancarboxylic acid, 100Kg of dichloromethane, and 5Kg of DMF into the dry reaction kettle, slowly pump in 160Kg of thionyl chloride, keep stirring at 20-30°C for 3 hours, and absorb the tail gas with liquid caustic soda (that is, liquid sodium hydroxide) , then heated up to 50°C, concentrated under reduced pressure, when the vacuum no longer increased, pumped in 50Kg of dichloromethane respectively, and continued to concentrate three times; after concentrating, added 270Kg of dichloromethane, lowered to room temperature, and put it in a barrel for future use. One-step reaction, optical purity: 99.3%;
[0038] (2) Preparation of Condensate (II)
[0039] Add 270Kg of dichloromethane and 132Kg of isopropylidene malonate to the reaction kettle, stir and cool d...
Embodiment 2
[0043] A kind of industrial preparation method of high optical purity S-acetyl tetrahydrofuran, comprises the following steps:
[0044] (1) Preparation of S-tetrahydrofuroyl chloride (Ⅲ)
[0045] Add 100Kg of S-tetrahydrofurancarboxylic acid, 120Kg of toluene, and 4Kg of pyridine into the dry reaction kettle, slowly pump in 200Kg of phosphorus oxychloride, keep stirring at 20-40°C for 4 hours, and absorb the tail gas with liquid caustic soda (that is, liquid sodium hydroxide) , then heated up to 50°C, concentrated under reduced pressure, when the vacuum no longer increased, sucked in 50Kg toluene respectively, and continued to concentrate three times; Purity: 99.3%;
[0046] (2) Preparation of Condensate (II)
[0047] Add 270Kg of toluene and 132Kg of isopropylidene malonate to the reaction kettle, stir and cool down to 5-10°C, add 255Kg of triethylamine, after the addition is complete, add the prepared S-tetrahydrofuran dropwise at -5-0°C The toluene solution of formyl chl...
Embodiment 3
[0051] A kind of industrial preparation method of high optical purity R-acetyl tetrahydrofuran, comprises the following steps:
[0052] (1) Preparation of R-tetrahydrofuroyl chloride (Ⅲ)
[0053] Add 100Kg of R-tetrahydrofurancarboxylic acid, 100Kg of tetrahydrofuran, and 5Kg of DMF into the dry reaction kettle, slowly suck in 160Kg of thionyl chloride, keep stirring at 20-30°C for 3 hours, absorb the tail gas with liquid caustic soda (that is, liquid sodium hydroxide), and then Raise the temperature to 50°C, concentrate under reduced pressure, and when the vacuum no longer increases, suck in 50Kg of dichloromethane, and continue to concentrate three times. After concentrating, add 270Kg tetrahydrofuran, cool down to room temperature, put it in barrels for the next reaction, optical purity: 99.4%;
[0054] (2) Preparation of Condensate (II)
[0055] Add 270Kg of tetrahydrofuran and 132Kg of isopropylidene malonate to the reaction kettle, stir and cool down to 5-10°C, add 273...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com