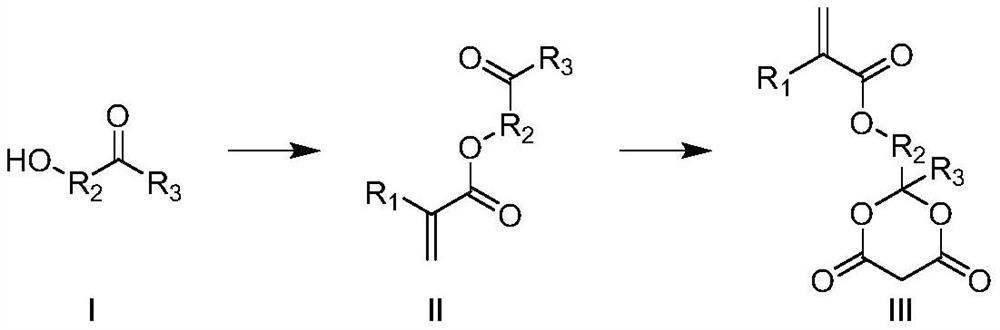
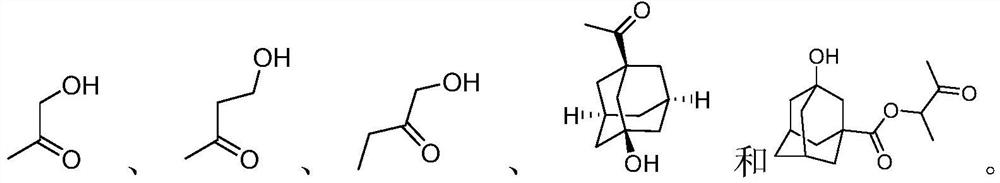
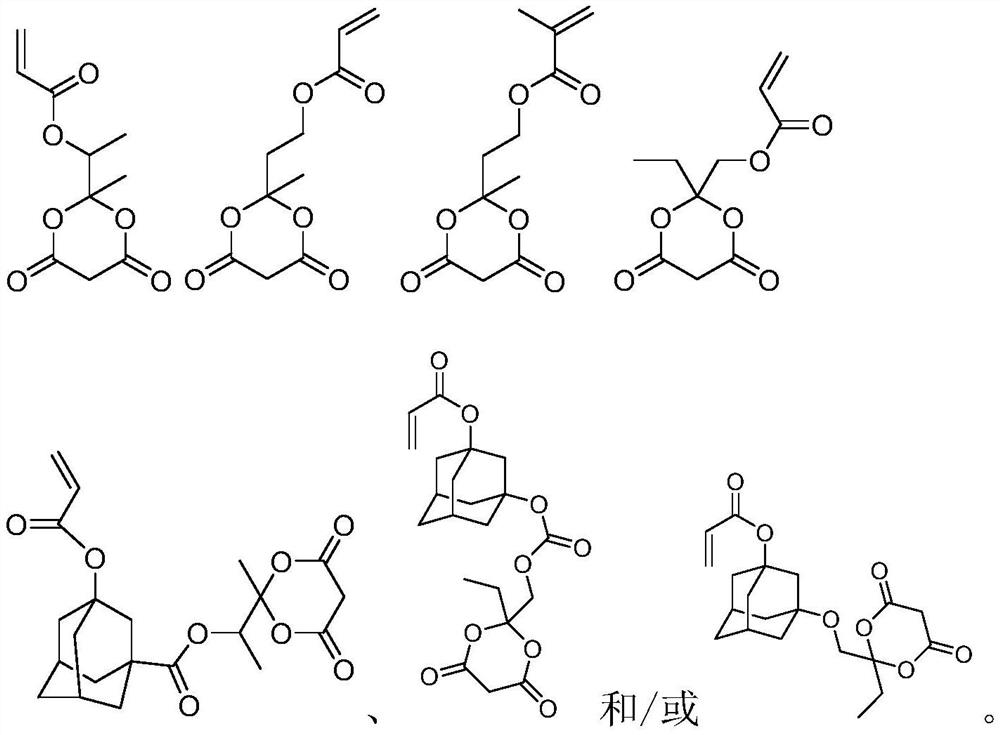
Photoresist resin monomer containing Meldrum's acid structure and synthesis method thereof
A technology of resin monomer and synthesis method, which is applied in the field of photoresist resin monomer containing Mie's acid structure and its synthesis, can solve the problems of reduced etching resistance, higher price, etc. Etching resistance, the effect of increasing alkali solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Preparation of Resin Monomers 1-3
[0026] The synthetic route is as follows:
[0027]
[0028] Reaction steps:
[0029] 3-Hydroxy-2-butanone 1-1 (20 g, 227 mmol) was added to dichloromethane (500 mL), triethylamine (45 g, 445 mmol) was added, the ice water was cooled to 0 °C, and acryloyl chloride ( 25g, 276mmol), warmed to room temperature and stirred for 3 hours, quenched by adding water (250mL) under ice-water cooling, the aqueous phase was extracted three times with dichloromethane (100mL×3), the combined organic phases were washed with saturated brine, anhydrous sodium sulfate After drying, concentrated in vacuo to obtain compound 1-2 (29.8 g, 210 mmol, yield: 92.4%).
[0030] Malonic acid (22 g, 211 mmol) and acetic anhydride (25.6 g, 251 mmol) were added to the reaction flask, concentrated sulfuric acid (1.8 g, 18.4 mmol) was added under ice-water bath cooling, stirred until malonic acid was dissolved, and slowly added dropwise Compound 1-2 (29.8 g, 210 mm...
Embodiment 2
[0032] Synthesis of Resin Monomers 2-4
[0033] The synthetic route is as follows:
[0034]
[0035] 3-Hydroxyadamantane-1-carboxylic acid 2-1 (20 g, 102 mmol) was added to dichloromethane (300 mL), triethylamine (21 g, 208 mmol) was added, and acryloyl chloride ( 10 g, 110 mmol), warmed to room temperature and stirred for 3 hours, under ice-water cooling, added water (300 mL) to quench, the aqueous phase was extracted with dichloromethane (150 mL×3), the organic phases were combined, washed with saturated brine, and concentrated in vacuo to obtain Compound 2-2 (23.1 g, 92 mmol, yield: 90.6%);
[0036] Compound 2-2 (23.1 g, 92 mmol) and 3-hydroxy-2-butanone (8.2 g, 93 mmol) were added to toluene (250 mL), p-toluenesulfonic acid (2 g, 12 mmol) was added, and the mixture was heated to reflux for 16 hours, Water (150 mL) was added to the reaction solution, the layers were separated, the aqueous phase was extracted with ethyl acetate (80 mL×3), the organic phases were combine...
Embodiment 3
[0039] Synthesis of Resin Monomers 3-4
[0040]
[0041] The synthetic route is as follows:
[0042] 1,3-adamantanediol 3-1 (20 g, 119 mmol) was added to dichloromethane (350 mL), triethylamine (24 g, 237 mmol) was added, and acryloyl chloride (12.9 g) was slowly added dropwise in an ice-water bath. , 143 mmol), rose to room temperature and stirred for 3 hours, quenched by adding water (200 mL) under ice-water bath cooling, the aqueous phase was extracted with dichloromethane (100 mL×3), the organic phases were combined and washed with saturated brine, and concentrated in vacuo to obtain Compound 3-2 (24 g, 108 mmol, yield: 90.9%).
[0043] Compound 3-2 (24 g, 108 mmol) was added to dichloromethane (300 mL), followed by carbonyldiimidazole (17.6 g, 109 mmol), stirred at 40°C for 3 hours, concentrated, added acetonitrile (300 mL), and then added 1- Hydroxy-2-butanone (9.5 g, 108 mmol) was continuously stirred at room temperature for 3 hours, the reaction solution was conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com