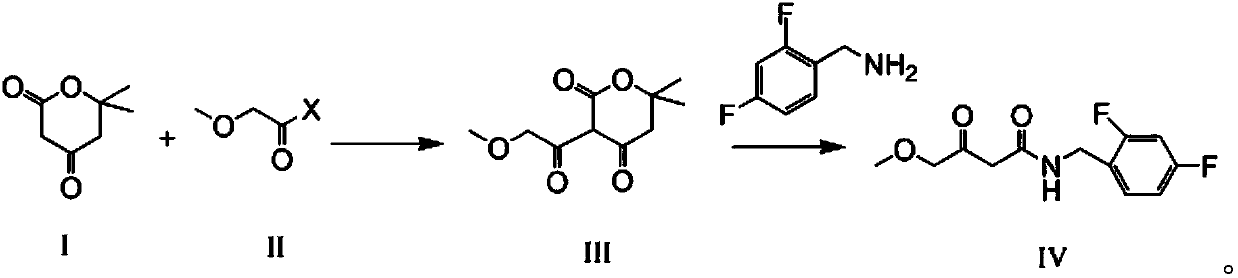
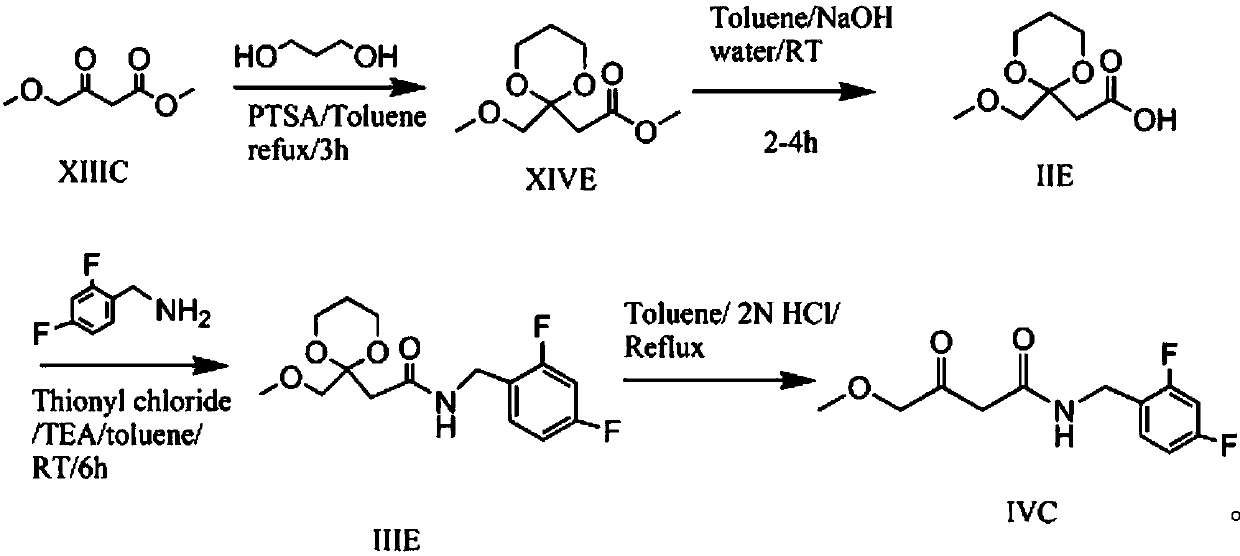
Preparation method of N-(2,4-fluorobenzoyl)-4-methoxyl-3-oxobutyrylamide
A technology of oxobutyramide and fluorobenzoyl, which is applied in the field of preparation of N--4-methoxy-3-oxobutanamide, can solve the problems of harsh reaction conditions, long reaction steps, difficult industrialized operation and the like , to achieve the effect of simple and easy-to-obtain raw materials, few reaction processes, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Add 14.2g cyclo(ethylene)isopropyl malonate (Formula I), 300ml dichloromethane, and 20.2g triethylamine into a 500mL reaction flask, stir and cool to 0-10°C, add dropwise 13g methoxyethyl Acyl chloride, rise to room temperature reaction after dropwise addition, until the raw material reaction is complete;
[0055] Then, filter, the organic phase is washed 2 times with 100g water, discard the water layer, and the organic layer is concentrated to dryness to obtain 21.3g of crude product comprising the compound of formula III, and the crude yield of the product is 100%, which can be directly used in the next step without purification operate.
Embodiment 2
[0057] Add 14.2g of cyclo(ethylene)isopropyl malonate (formula I), 200ml of tetrahydrofuran, 20.2g of N-methylmorpholine into a 500mL reaction flask, stir and cool to 0-10°C, add dropwise 20g of methoxyacetic anhydride , After the dropwise addition is completed, it is raised to room temperature and reacted until the reaction of the raw materials is completed;
[0058] Then, filter, concentrate the organic layer to dryness, add 300ml of dichloromethane for extraction, wash the organic phase twice with 100g water, discard the water layer, concentrate the organic layer to dryness, and recrystallize to obtain 18.2g of the compound of formula III, with a yield of 85.0% .
Embodiment 3
[0060] Add 14.2g of cyclo(ethylene)isopropyl malonate (Formula I) and 200ml of tetrahydrofuran into a 500mL reaction flask, stir and cool to 0-10°C, add 8.2g of sodium methoxide, and then dropwise add 13.5g of methyl methoxyacetate Esters, after the dropwise addition, rise to room temperature and react until the reaction of the raw materials is complete;
[0061] Then, add 5% hydrochloric acid dropwise to adjust the pH to neutral, add 300ml of toluene for extraction, wash the organic phase twice with 100g of water, discard the water layer, concentrate the organic layer to dryness, and recrystallize to obtain 16.8g of the compound of formula III. 78.5%.
[0062] Preparation of N-(2,4-fluorobenzoyl)-4-methoxy-3-oxobutanamide (compound of formula IV)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com