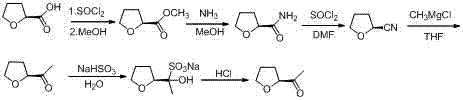
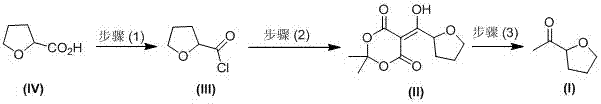
A kind of industrial preparation method of high optical purity acetyl tetrahydrofuran
A technology of acetyltetrahydrofuran and optical purity, which is applied in the field of industrialized preparation of high optical purity acetyltetrahydrofuran, can solve the problems of difficult product purification, complicated operation, low yield and the like, and achieves stable quality, low preparation cost and low raw material cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of industrial preparation method of high optical purity S-acetyl tetrahydrofuran, comprises the following steps:
[0036] (1) Preparation of S-tetrahydrofuroyl chloride (Ⅲ)
[0037] Add 100 Kg of S-tetrahydrofurancarboxylic acid, 100 Kg of dichloromethane, and 5 Kg of DMF into the dry reaction kettle, slowly suck in 160 Kg of thionyl chloride, keep stirring at 20-30°C for 3 h, and use liquid caustic soda (that is, liquid state Sodium hydroxide) absorption, then warmed up to 50 ℃, concentrated under reduced pressure, when the vacuum no longer increased, pumped in 50 Kg of dichloromethane, and continued to concentrate three times; after concentration, added 270 Kg of dichloromethane, reduced to At room temperature, in barrels for the next reaction, optical purity: 99.3%;
[0038] (2) Preparation of Condensate (II)
[0039] Add 270 Kg of dichloromethane and 132 Kg of isopropylidene malonate to the reaction kettle, stir and cool down to 5-10°C, add 265 Kg of pyridi...
Embodiment 2
[0043] A kind of industrial preparation method of high optical purity S-acetyl tetrahydrofuran, comprises the following steps:
[0044] (1) Preparation of S-tetrahydrofuroyl chloride (Ⅲ)
[0045] Add 100 Kg of S-tetrahydrofurancarboxylic acid, 120 Kg of toluene, and 4 Kg of pyridine into the dry reaction kettle, slowly pump in 200 Kg of phosphorus oxychloride, keep stirring at 20-40°C for 4 h, and use liquid caustic soda (that is, liquid hydrogen Sodium oxide) to absorb, then heat up to 50 °C, concentrate under reduced pressure, when the vacuum no longer increases, pump in 50 Kg of toluene respectively, and continue to concentrate three times; Prepare for the next reaction, optical purity: 99.3%;
[0046] (2) Preparation of Condensate (II)
[0047] Add 270 Kg of toluene and 132 Kg of isopropylidene malonate to the reaction kettle, stir and cool down to 5-10°C, add 255 Kg of triethylamine, after the addition is complete, add the prepared solution dropwise at -5-0°C while cont...
Embodiment 3
[0051] A kind of industrial preparation method of high optical purity R-acetyl tetrahydrofuran, comprises the following steps:
[0052] (1) Preparation of R-tetrahydrofuroyl chloride (Ⅲ)
[0053] Add 100 Kg of R-tetrahydrofurancarboxylic acid, 100 Kg of tetrahydrofuran, and 5 Kg of DMF into the dry reaction kettle, slowly suck in 160 Kg of thionyl chloride, keep stirring at 20-30°C for 3 h, and use liquid caustic soda (that is, liquid hydrogen Sodium Oxide) absorption, and then warmed up to 50 ° C, concentrated under reduced pressure, when the vacuum no longer increased, pumped in 50 Kg of dichloromethane, and continued to concentrate three times. After concentrating, add 270 Kg of tetrahydrofuran, drop to room temperature, and put it in barrels for the next reaction, optical purity: 99.4%;
[0054] (2) Preparation of Condensate (II)
[0055] Add 270 Kg of tetrahydrofuran and 132 Kg of isopropylidene malonate to the reaction kettle, stir and cool down to 5-10°C, add 273 Kg o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com