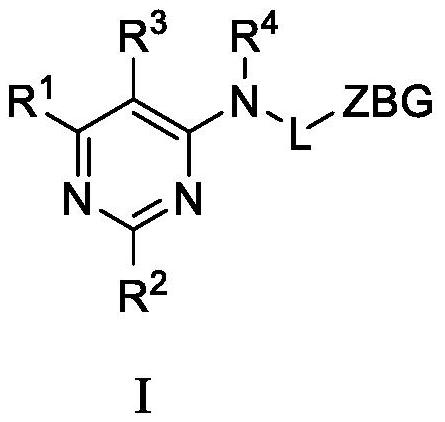
Substituted pyrimidine compound, and preparation method, intermediate and application thereof
A technology of pyrimidines and compounds, applied in the field of substituted pyrimidines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
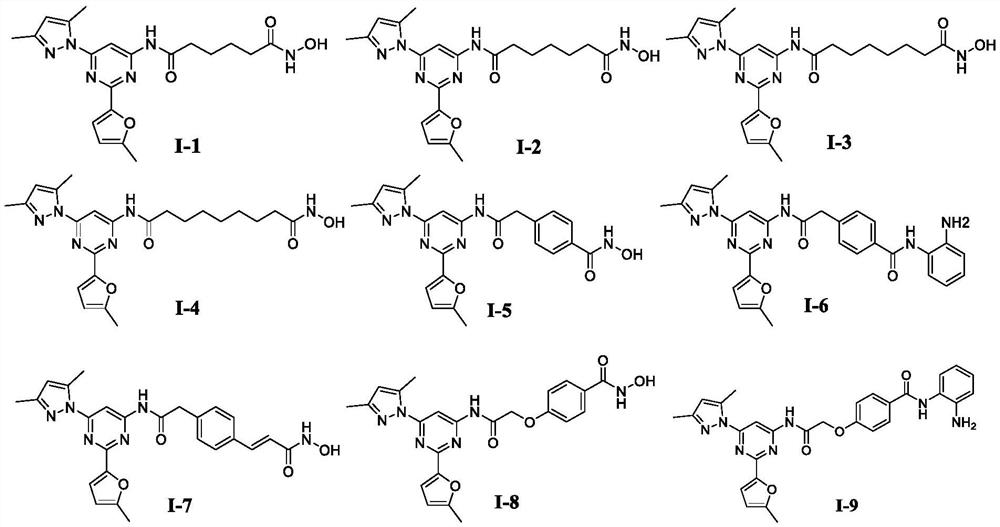
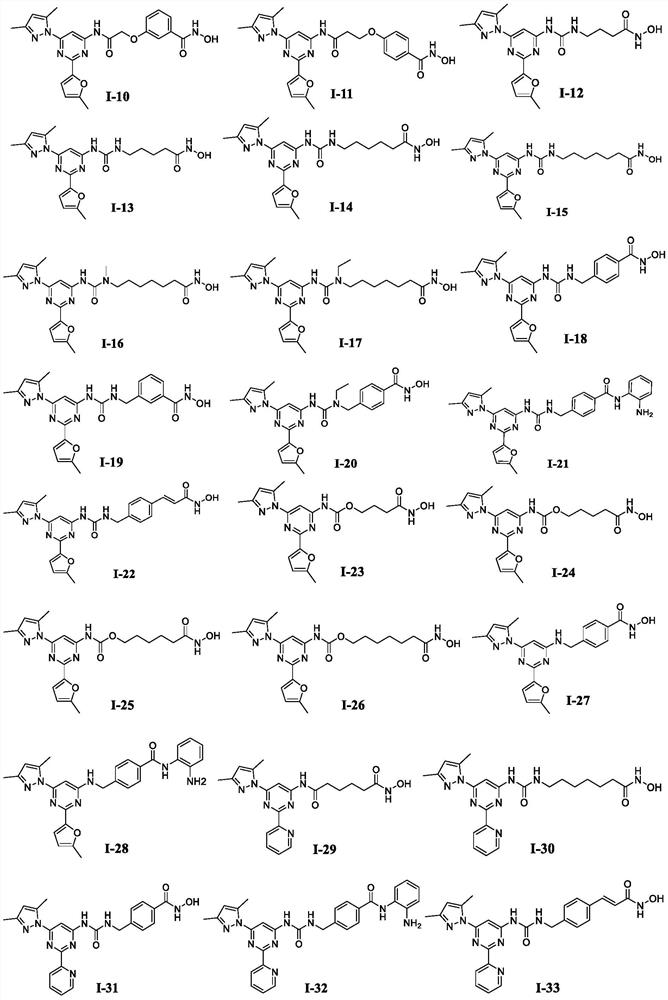
[0245] Example 1: N 1 -(6-(3,5-Dimethyl-1H-pyrazol-1-yl)-2-(5-methylfuran-2-yl)pyrimidin-4-yl)-N 6 -Hydroxyadipamide (Compound I-1)
[0246]
[0247] Step 1: Refer to literature (Slee et al., J Med Chem 2008, 51, 1719-1729) to prepare intermediate INT-1: 6-(3,5-dimethyl-1H-pyrazol-1-yl)-2 -(5-Methylfuran-2-yl)-4-aminopyrimidine. After dissolving monomethyl adipate (432 mg, 2.70 mmol) in dichloromethane (10 mL), benzotriazole (650 mg, 5.40 mmol) and thionyl chloride (0.40 mL, 5.40 mmol) were added successively, and the reaction was carried out at room temperature. 1 hour. The reaction solution was filtered, and the filtrate was evaporated under reduced pressure to obtain an orange-yellow oily liquid. After dissolving INT-1 (122 mg, 0.45 mmol) in dichloromethane (5 mL), pyridine (0.5 mL) was added successively, and the above obtained orange-yellow oily liquid was stirred at room temperature for 12 hours. After the reaction was completed, water (5 mL) was added to dilute,...
Embodiment 2
[0251] Example 2: N 1 -(6-(3,5-Dimethyl-1H-pyrazol-1-yl)-2-(5-methylfuran-2-yl)pyrimidin-4-yl)-N 7 -Hydroxypimelamide (Compound I-2)
[0252]
[0253] Using the same method as in Example 1, replacing "monoethyl adipate" with "monomethyl pimelic acid", compound I-2 can be prepared as a white solid. HRMS(ESI)C 21 H 27 N 6 O 4 + [M+H] + Calculated value: 427.2088, measured value: 427.2091; 1 H NMR (600MHz, DMSO-d 6 )δ11.05(s,1H),10.34(s,1H),8.35(s,1H),7.19(d,J=3.3Hz,1H),6.35(dd,J=3.3,1.2Hz,1H), 6.21(s, 1H), 2.72(s, 3H), 2.45(t, J=7.3Hz, 2H), 2.38(s, 3H), 2.22(s, 3H), 1.94(t, J=7.4Hz, 2H) ), 1.60-1.54 (m, 2H), 1.53-1.48 (m, 2H), 1.29-1.25 (m, 2H); HPLC: 96.9%.
Embodiment 3
[0254] Example 3: N 1 -(6-(3,5-Dimethyl-1H-pyrazol-1-yl)-2-(5-methylfuran-2-yl)pyrimidin-4-yl)-N 8 -Hydroxysuberamide (Compound I-3)
[0255]
[0256]Using the same method as in Example 1, replacing "monoethyl adipate" with "monomethyl suberate", compound I-3 can be prepared as a white solid. HRMS(ESI)C 22 H 29 N 6 O 4 + [M+H] + Calculated value: 441.2245, measured value: 441.2250; 1 H NMR (800MHz, DMSO-d 6 )δ11.02(s,1H),10.32(s,1H),8.35(s,1H),7.20–7.14(m,1H),6.35(dd,J=3.2,1.0Hz,1H),6.20(s ,1H),2.72(s,3H),2.45(t,J=7.4Hz,2H),2.38(s,3H),2.22(s,3H),1.94(t,J=7.4Hz,2H),1.60 - 1.54 (m, 2H), 1.52 - 1.45 (m, 2H), 1.31 - 1.24 (m, 4H); HPLC: 96.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com