Method for synchronously removing chlorite and organic pollutants in water by combining sulfite and inorganic peroxide
A technology of inorganic peroxides and organic pollutants, applied in the field of water treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
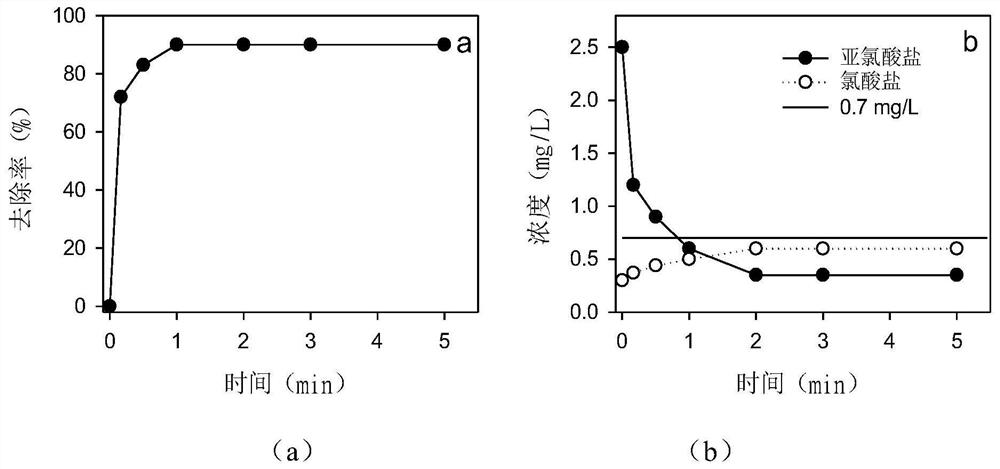
[0030] The water to be treated is slightly polluted source water, in which TOC is 6.8mg / L, dissolved oxygen is 5.9mg / L, and initial pH is 8.2. After pre-oxidation treatment with chlorine dioxide (4.0mg / L), the concentration of chlorite The concentration of chlorate is 2.5mg / L, the concentration of chlorate is 0.3mg / L, and the concentration of target pollutant naproxen is 1.0mg / L.
[0031] The present embodiment is carried out as follows: adjust the pH value of the above-mentioned water to be treated to 6.0, first add hydrogen peroxide (25mg / L) and fully stir, feed high-purity oxygen, so that the dissolved oxygen concentration is 7.9mg / L, Then 70mg / L sodium sulfite was added, the reaction was stirred and samples were taken to test the concentration of remaining naproxen, chlorite and chlorate. The result is as figure 1 Shown, when reacting 1min, the removal rate of naproxen reaches more than 90% ( figure 1 a). When the reaction time reaches 2min, the removal of chlorite can ...
Embodiment 2
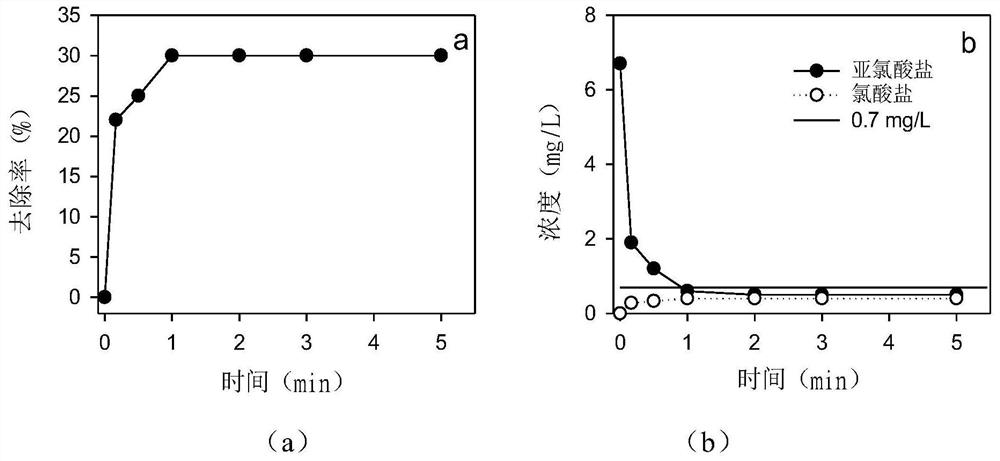
[0033] The water to be treated is a simulated water sample of aniline wastewater, in which the concentration of aniline is 10 mg / L, the initial pH is 5.0, the concentration of dissolved oxygen is 6.8 mg / L, and the concentration of chlorite is 6.7 mg / L.
[0034] The present embodiment is carried out as follows: add calcium peroxide (15mg / L) to the above-mentioned water to be treated and fully stir, feed high-purity oxygen so that the dissolved oxygen concentration is 8.0mg / L, then add sodium sulfite, the initial concentration of sodium sulfite 200mg / L, stirred and reacted for 10min, sampled and tested the remaining aniline, chlorite, and chlorate concentration. like figure 2 Shown, when reacting 1min, the removal rate of aniline is 30% ( figure 2 a), the concentration of residual sodium chlorite is 0.5mg / L, and the concentration of chlorate generated is 0.4mg / L ( figure 2 b), all meet the limit requirements (0.7mg / L) in my country's "Urban Water Supply Quality Standards". R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com