Application of gene marker combination in diagnosis of endometrial cancer
A technology for endometrial cancer and biomarkers, which is applied in the determination/examination of microorganisms, biochemical equipment and methods, and instruments, etc., can solve the problems of low sensitivity, difficulty in screening patients, and inability to predict recurrence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Screening of biomarkers related to endometrial cancer
[0073] 1. Screening method
[0074] (1) The data used for screening and preprocessing methods
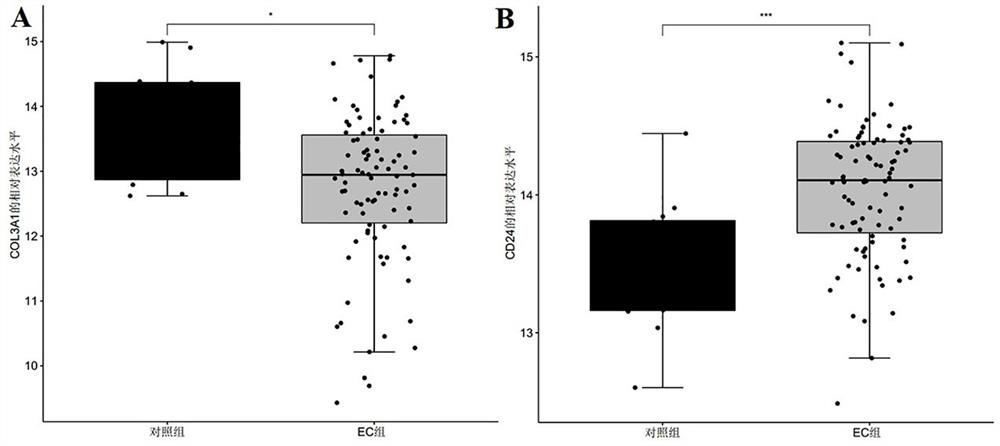
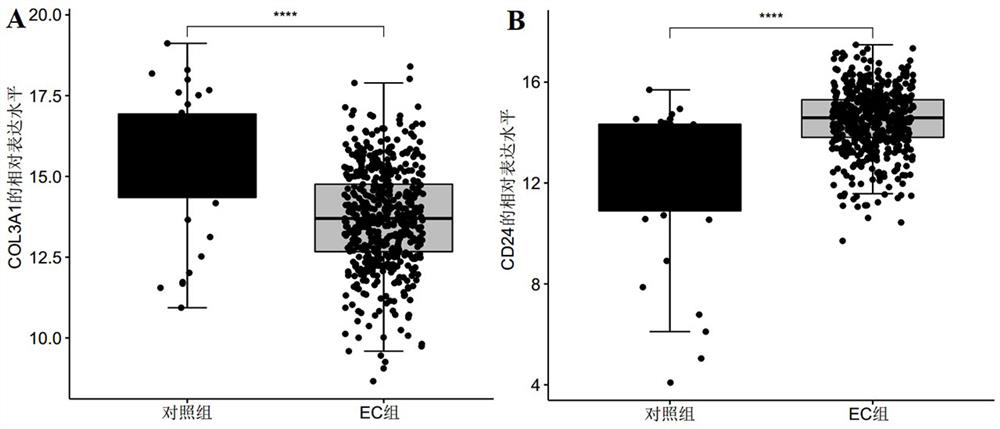
[0075] In this example, the data for screening biomarkers related to endometrial cancer were downloaded from the GEO database and the TCGA database respectively, and the public gene expression data and complete clinical Note, among them, the microarray data and clinical information of the GSE17025 dataset were downloaded from the GEO database as a training set, and the sample size was endometrial cancer group: control group = 91:12; the RNA of endometrial cancer was downloaded from the TCGA database- The seq data and clinical information are used as the verification set, and the sample size is endometrial cancer group: control group = 543:35;
[0076] The fastp software double-end sequence automatic detection mode is used to process the raw data; the minimum N base number threshold is 5, the reads minimum len...
Embodiment 2
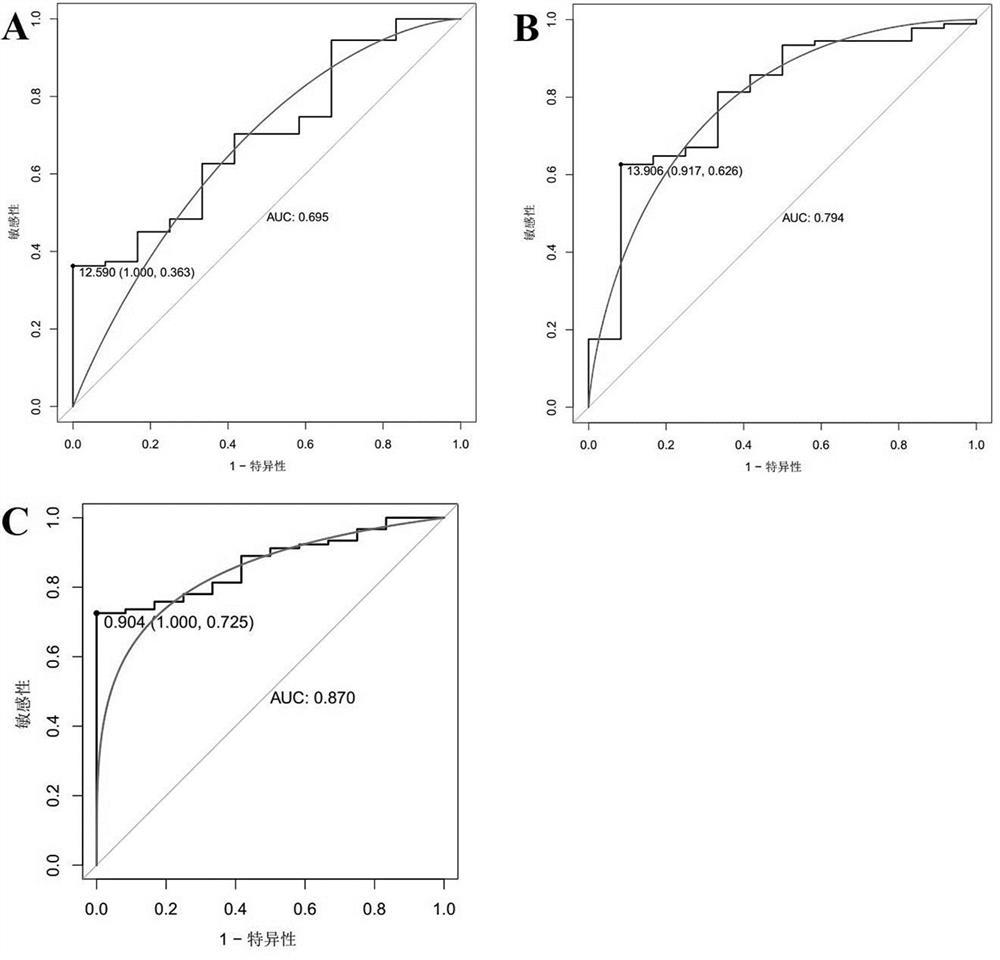
[0083] Example 2 Validation and analysis of the diagnostic efficacy of the screened biomarkers
[0084] 1. Verify the analysis method
[0085] The R package "pROC" was used to draw the receiver operating curve (ROC), and the biomarkers COL3A1, CD24, and COL3A1 that were screened in Example 1 and showed significant differential expression between the control group and the endometrial cancer group were analyzed The AUC value, sensitivity and specificity of +CD24 were used to judge its diagnostic efficacy for endometrial cancer. Among them, when evaluating and analyzing the diagnostic efficacy of single biomarkers COL3A1 and CD24 for endometrial cancer, the expression level of the gene (Log 2 expression level) for evaluation and analysis, select the point corresponding to the largest Youden index as its cutoff value, that is, the optimal division threshold is determined by the point with the largest Youden index; in the evaluation and analysis of the combined biomarker COL3A1+CD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com