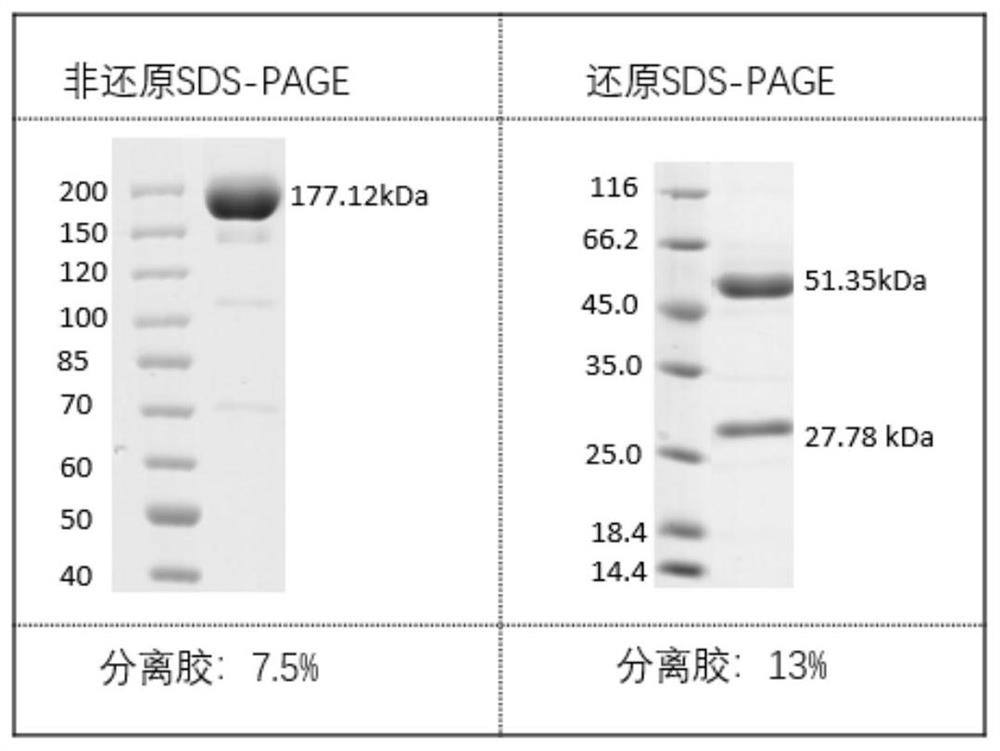
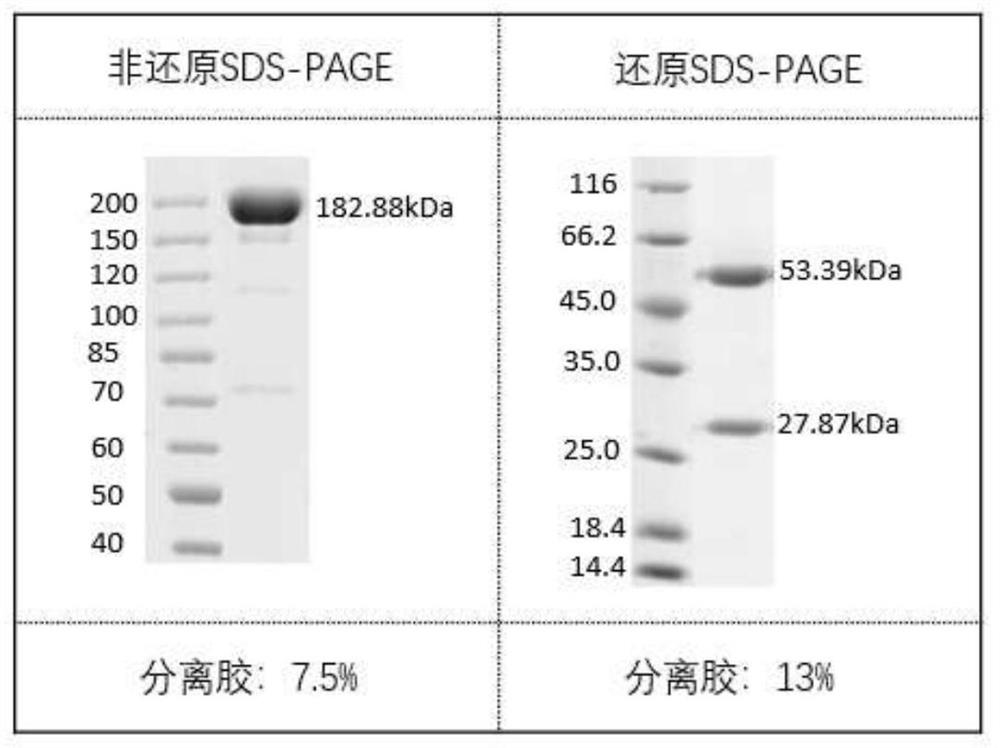
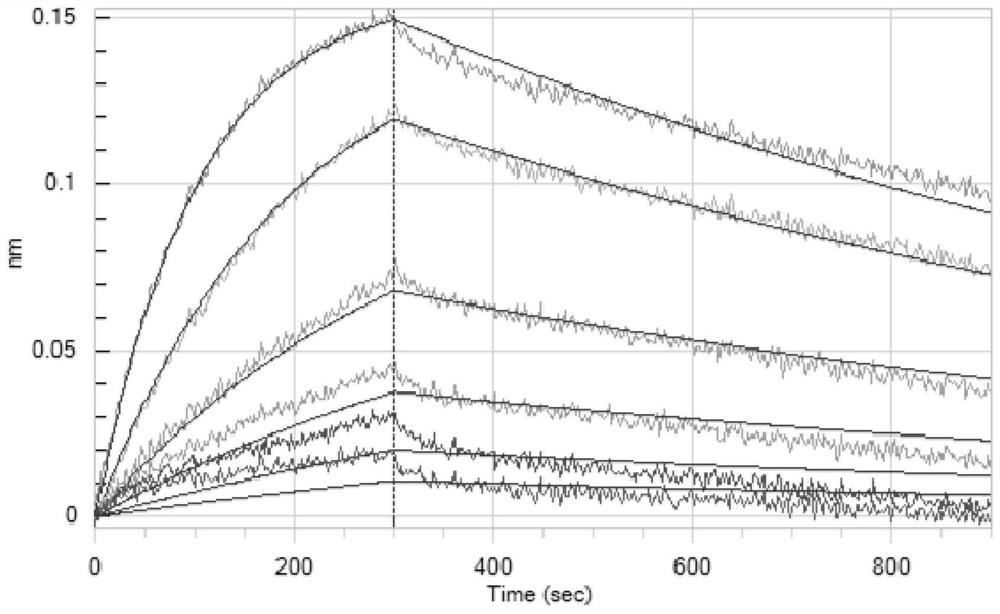
Antibodies against SARS-CoV-2 mutants and uses thereof
A technology of antibodies and variants, applied in the direction of antibodies, applications, antiviral agents, etc., can solve problems such as the impact of vaccine effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Example 1: Preparation of Antibodies BD812 and BD836
[0153] 1.1 Obtaining antibody sequences
[0154] 1) Isolation of B cells
[0155] The blood (provided by Beijing You'an Hospital) was collected from persons infected with SARS-CoV-2 virus and cured for more than half a year, and peripheral blood mononuclear cells were extracted. Utilize STEMCELL EasySep TM Human BCell Isolation Kit (STEMCELL, 17954) isolates B cells from peripheral blood mononuclear cells.
[0156] 2) Enrichment and sequencing of antigen-specific B cells
[0157] Combining antigenic proteins (RBD, S1, S2) with DNA oligonucleotides and fluorescent streptavidin into a complex: combine RBD (Sino Biological 40592-V27H-B) with TotalSeq TM -C0951 PE Streptavidin (Biolegend 405261) was assembled into a complex according to the manufacturer's instructions. Similarly, S1 (Sino Biological40591-V27H-B) and TotalSeq TM -C0952 PE Streptavidin (Biolegend 405263), S2 (SinoBiological 40590-V08B-B) TotalSeq TM -...
Embodiment 2
[0163] Example 2: Identification of Antigen Binding Ability of Antibodies BD812 and BD836
[0164] Using recombinantly expressed RBD (Sinobiologicals, Cat: 40592-V08B, which contains RBD fragment sequence YP_009724390.1 and His tag) as the coating antigen, horseradish peroxidase (HRP)-labeled Goat Anti-Human IgG (H +L) (Jackson, 109-036-088) was used as the secondary antibody, and the reaction specificity of the purified antigen was detected by ELISA experiment. Briefly, a 96-well plate was coated with the recombinantly expressed S protein RBD (the amino acid sequence of which is SEQ ID NO: 41), and then the 96-well plate was blocked with a blocking solution. Then, monoclonal antibodies to be tested (irrelevant control antibodies, BD812, BD836) were added and incubated. After washing with ELISA washing solution, horseradish peroxidase-labeled Goat Anti-Human IgG (H+L) was added as a secondary antibody (diluted at 1:5000), and the incubation was continued. Wash the ELISA plat...
Embodiment 3
[0170] Example 3: Evaluation of Antibody BD812 and BD836 Neutralizing Pseudovirus Ability
[0171] The SARS-CoV-2 pseudovirus (mutant strain pseudovirus) used in this experiment was provided by the China Institute for Food and Drug Control. This pseudovirus can simulate the similar cell infection characteristics of the real virus, and carries a fluorescein reporter gene for detection. For the experimental steps, please refer to: Nie, J., Li, Q., Wu, J. et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat Protoc 15, 3699–3715(2020). https: https: / / doi.org / 10.1038 / s41596-020-0394-5. Specific steps are as follows:
[0172] 1) The arrangement of the samples in the 96-well plate is as follows: B2-G2 is the cell control well (CC), containing Huh-7 cells without antibody samples and pseudoviruses; B3-G3 is the virus control well (VC), containing Huh-7 cells and pseudoviruses, without antibody samples; wells B4-G11 are experimental wells,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com