7-methoxy-1h-indole compound, preparation method, pharmaceutical composition and application
A compound, methoxy technology, applied in the field of 7-methoxy-1H-indole compounds, can solve the problems of low oral bioavailability of peptide compounds, limited clinical application, etc., and achieve great application value and side effects. Small, high-yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
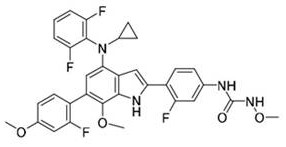
[0058] Compounds as shown in Formula 1: 1-(4-(4-(cyclopropyl(2,6-difluorophenyl)amino)-6-(2-fluoro-4-methoxyphenyl)-7 -methoxy-1H-indol-2-yl)-3-fluorophenyl)-3-methoxyurea; its synthetic reaction formula is as follows:
[0059]
[0060] The first step: compound 1a (113.7g, 300.0mmol), compound 1b (50.7g, 300.0mmol), Pd(dppf)Cl 2 (11.0g, 15.0mmol), potassium carbonate (92.9g, 720mmol) were dissolved in DMF (800mL), heated to 100°C for 10 hours, and the reaction was monitored by TLC. After the reaction was completed, water (500mL) was added to quench the reaction. Ethyl ester (500 mL×2) was extracted, the organic layers were combined, dried, concentrated, and separated by column chromatography to obtain 106.4 g of a light yellow solid (compound 1c), with a yield of 75.8%. Wherein, the structural formula of compound 1a is shown in formula 1a, and other compounds can be deduced in the same way.
[0061] The second step: compound 1c (106.0g, 226.4mmol), compound 1d (38.5g, 226...
Embodiment 2
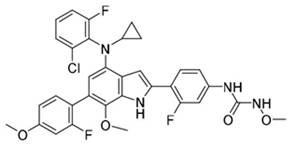
[0068] Compound shown in formula 2: 1-(4-(4-((2-chloro-6-fluorophenyl) (cyclopropyl) amino)-6-(2-fluoro-4-methoxyphenyl )-7-methoxy-1H-indol-2-yl)-3-fluorophenyl)-3-methoxyurea; its synthetic reaction formula is as follows:
[0069]
[0070] The first step: compound 1a (37.9g, 100.0mmol), compound 2a (18.5g, 100.0mmol), Pd(dppf)Cl 2 (3.7g, 5.0mmol), potassium carbonate (20.7g, 150.0mmol) were dissolved in DMF (200mL), and the temperature was raised to 100° C. for 10 hours, and the reaction was monitored by TLC. After the reaction was completed, water (200mL) was added to quench the reaction. Extracted with ethyl acetate (200 mL×2), combined the organic layers, dried, concentrated, and separated by column chromatography to obtain 36.4 g of a light yellow solid (compound 2b), with a yield of 75.2%.
[0071] The second step: compound 2b (35.0g, 72.3mmol), compound 1d (12.3g, 72.3mmol), Pd(PPh 3 ) 4 (4.2g, 3.6mmol), cesium carbonate (35.1g, 108.5mmol) were dissolved in dioxa...
Embodiment 3
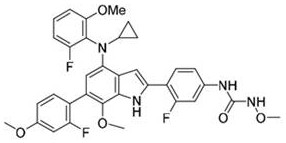
[0078]Compounds as shown in formula 3: 1-(4-(4-(cyclopropyl(2-fluoro-6-methoxyphenyl) amino)-6-(2-fluoro-4-methoxyphenyl )-7-methoxy-1H-indol-2-yl)-3-fluorophenyl)-3-methoxyurea; its synthetic reaction formula is as follows:
[0079]
[0080] The first step: compound 1a (37.9g, 100.0mmol), compound 3a (18.1g, 100.0mmol), Pd(dppf)Cl 2 (3.7g, 5.0mmol), potassium carbonate (20.7g, 150.0mmol) were dissolved in DMF (200mL), and the temperature was raised to 100° C. for 10 hours, and the reaction was monitored by TLC. After the reaction was completed, water (200mL) was added to quench the reaction. Extracted with ethyl acetate (200 mL×2), combined the organic layers, dried the organic layers, concentrated, and separated by column chromatography to obtain 34.5 g of a light yellow solid (compound 3b), with a yield of 71.9%.
[0081] The second step: compound 3b (24.0g, 50.0mmol), compound 1d (8.5g, 50.0mmol), Pd(PPh 3 ) 4 (2.9g, 2.5mmol), cesium carbonate (32.4g, 100.0mmol) were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com