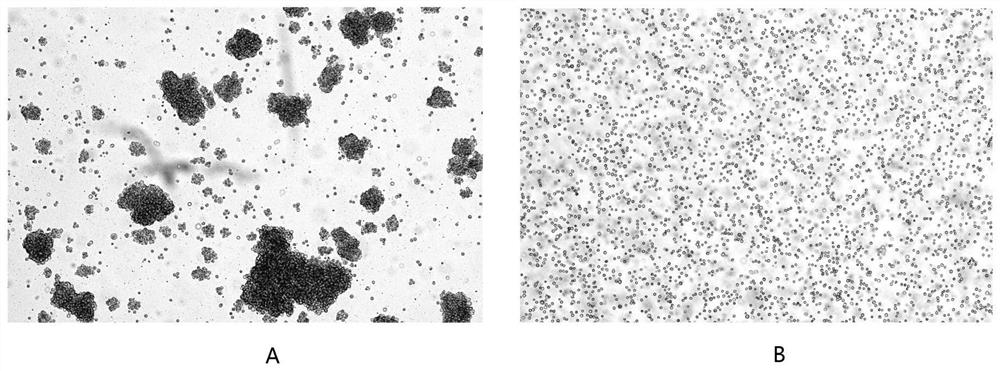
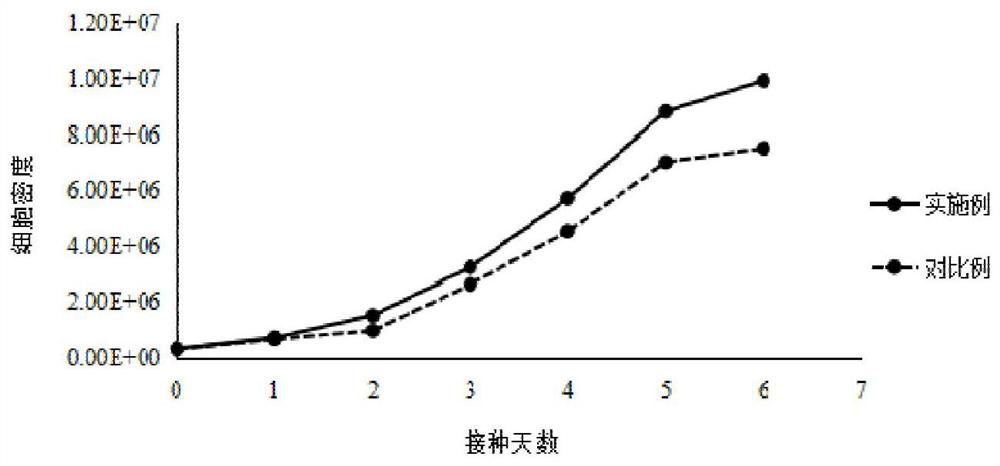
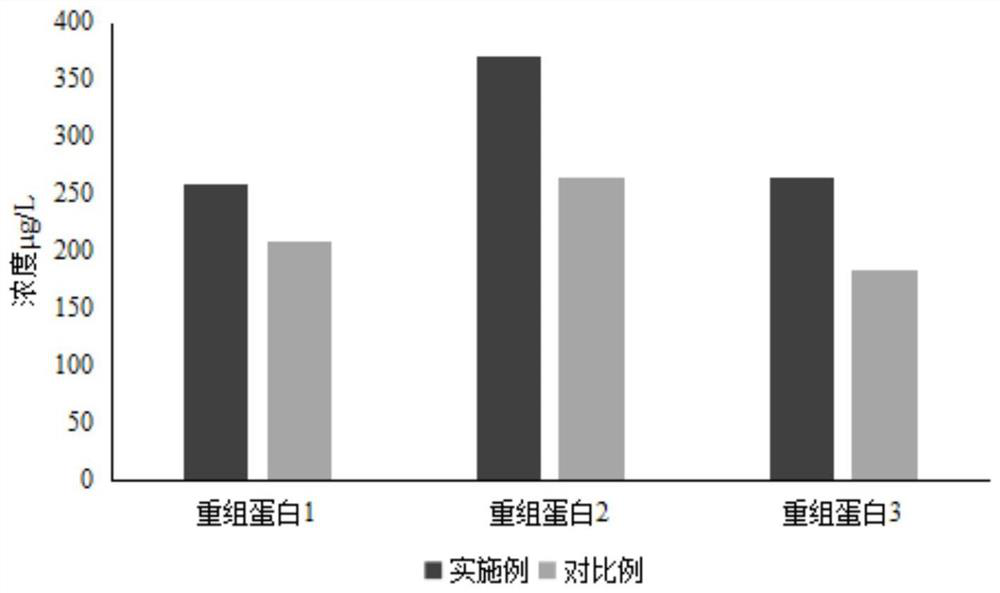
Serum-free culture medium suitable for HEK293 cells
A serum-free medium and medium technology, applied in the field of medium, can solve the problems of HEK293 cells that cannot be expanded at high density, continuous passage, affect cell viability, affect cell growth, etc., and achieve easy scale-up of culture and high protein production , reduce the effect of cell clumping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A serum-free medium suitable for HEK293 cells comprising amino acids, inorganic salts, vitamins, antisurbs and stabilizers. Each 1 L of medium contains 720 mg of amino acid, 25602.6 mg, vitamin 33.93 mg, additive 2615 mg, nucleoside 183 mg, F68600 mg, glutagro 200 mg, and the specific component and content are shown in Table 1.
[0029] The above components were subjected to aseptic deionized water, and the pH of the medium was adjusted with HCl and NaOH.
Embodiment 2
[0031] A serum-free medium suitable for HEK293 cells comprising amino acids, inorganic salts, vitamins, antisurbs and stabilizers.
[0032] Each 1 l was medium containing amino acid 1035 mg, inorganic salts 13310.9 mg, vitamin 62.5 mg, additive 1060 mg, nucleoside 587 mg, F68300 mg, Glutagro 400 mg, and water; specific components and content are shown in Table 1.
[0033] The above components were subjected to aseptic deionized water, and the pH of the medium was adjusted with HCl and NaOH to be 7.5.
Embodiment 3
[0035] A serum-free medium suitable for HEK293 cells comprising amino acids, inorganic salts, vitamins, antisurbs and stabilizers.
[0036] Each 1 l was medium containing amino acid 1235 mg, inorganic salts 18751.5 mg, vitamin 77.37 mg, additive 3250 mg, nucleoside 40 mg, F68800 mg, Glutagro 900 mg, and the remaining amount is water; specifically and the content is shown in Table 1.
[0037] The above components were subjected to sterile deionized water, and the pH of the medium was adjusted with HCl and NaOH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com