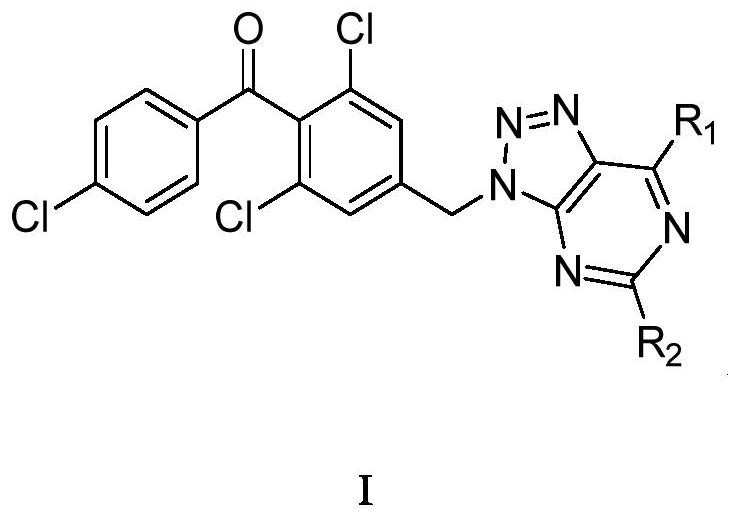
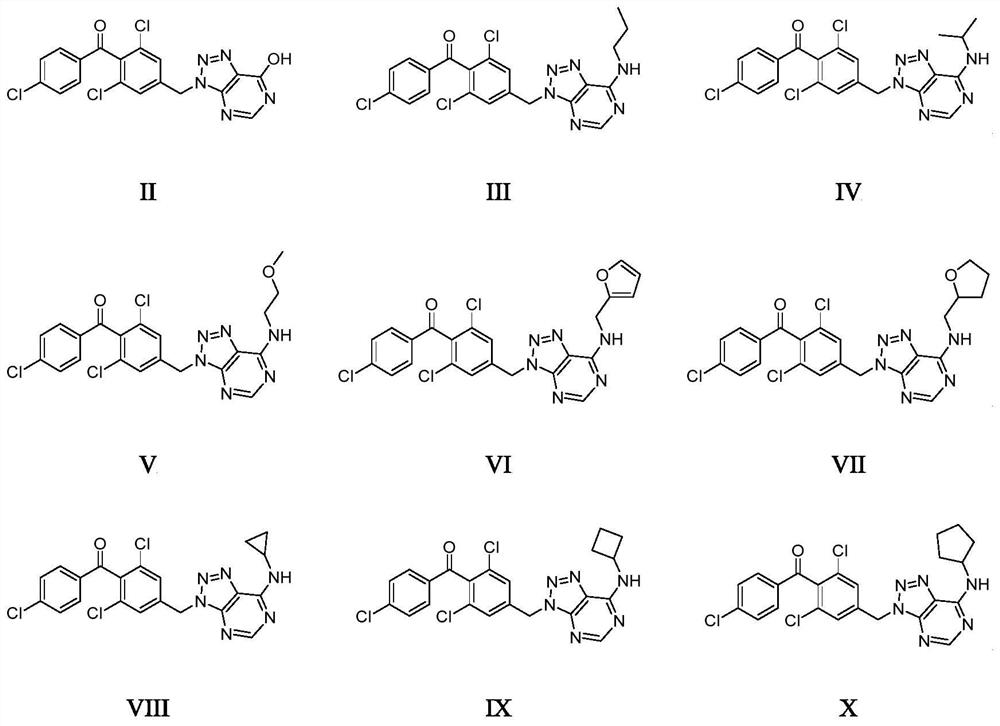
1, 2, 3-triazolopyrimidine compound and preparation method and application thereof
A compound, C1-C4 technology, applied in the direction of organic chemistry, drug combination, pharmaceutical formulation, etc., can solve the problem of lack of similar compounds and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 3-(3,5-dichloro-4-(4-chlorobenzoyl)benzyl)-3,6-dihydro-7H-[1,2,3]triazolo[4,5 -d] pyrimidin-7-one (B)
[0047]
[0048] Under nitrogen protection, carboxyamidotriazole (640 mg, 1.51 mmol), p-toluenesulfonic acid monohydrate (143.34 mg, 0.76 mmol) and trimethyl orthoformate (1602.4 mg, 15.1 mmol) were added into THF. The reaction solution was stirred at 90°C for 6 hours. Stop the reaction, cool to room temperature, add 5 mL of ethyl acetate, wash with water three times, dry the ethyl acetate layer with anhydrous sodium sulfate, filter, evaporate to dryness under reduced pressure, and the residue is separated by column chromatography (eluent petroleum ether: ethyl acetate 1:1), a white solid (0.498g, 76.2%) was obtained. 1 H NMR (400MHz, DMSO-d 6 ) δ 12.74 (s, 1H), 8.32 (s, 1H), 7.76 (s, 2H), 7.67 (s, 2H), 7.63 (s, 2H), 5.89 (s, 2H).
Embodiment 2
[0049] Example 2 (4-chlorophenyl) (2,6-dichloro-4-((7-(propylamino)-3H-[1,2,3]triazolyl[4,5-d]pyrimidine- 3-yl)methyl)phenyl)methanone (C1)
[0050]
[0051] Under nitrogen protection, 3-(3,5-dichloro-4-(4-chlorobenzoyl)benzyl)-3,6-dihydro-7H-[1,2,3]triazolo[ 4,5-d]pyrimidin-7-one (200mg, 0.46mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (265.5mg, 0.60mmol) and phenylhexafluorophosphate Triazol-1-oxytris(dimethylamino)phosphorus (106.6 mg, 0.70 mmol) was added to acetonitrile. The reaction was stirred at room temperature for 12 hours. The reaction was stopped, and acetonitrile was distilled off under reduced pressure. Add 5 mL of ethyl acetate, wash with water three times, dry the ethyl acetate layer with anhydrous sodium sulfate, filter, evaporate to dryness under reduced pressure, and separate the residue by column chromatography (eluent petroleum ether: ethyl acetate 2:1) , to obtain a white solid (0.160 g, 73.1%). 1 HNMR (400MHz, DMSO-d 6 )δ9.11(t, J=5.8Hz, 1H), 8.43(...
Embodiment 3
[0052] The synthesis of embodiment 3 compounds
[0053] With reference to the method of embodiment 2, prepare following formula compound:
[0054]
[0055] (4-Chlorophenyl)(2,6-dichloro-4-((7-(isopropylamino)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)methyl)phenyl)methanone . NMR data: 1 H NMR (400MHz, DMSO-d 6 )δ8.96(d, J=8.0Hz, 1H), 8.43(s, 1H), 7.77(d, J=8.6Hz, 2H), 7.65(d, J=7.9Hz, 4H), 5.90(s, 2H), 4.53 (dt, 1H), 1.28 (dd, J=19.1, 6.5Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com