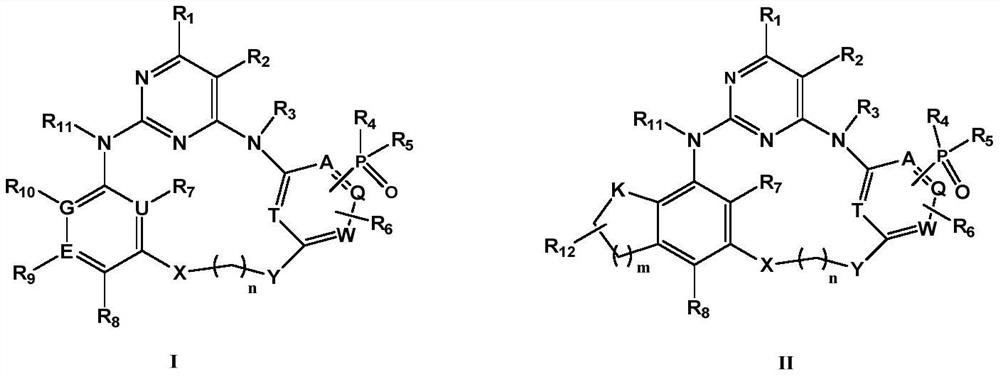
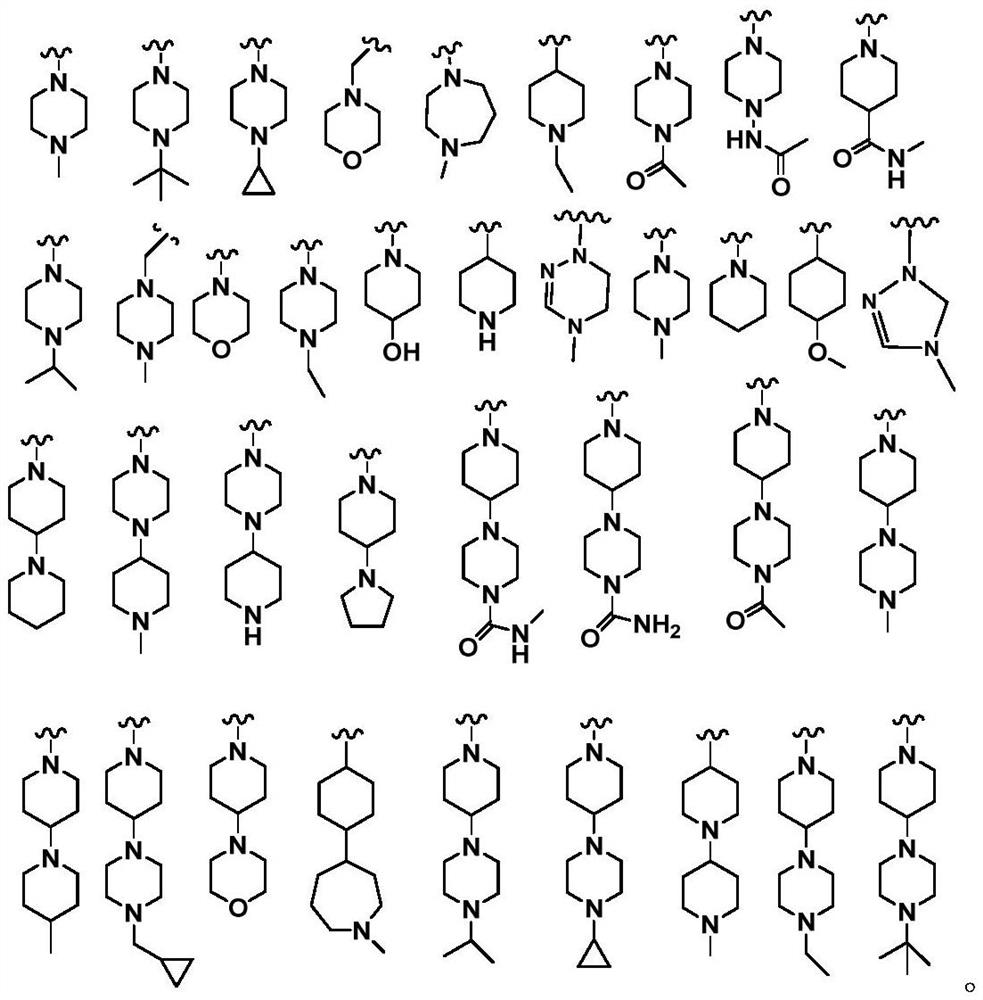
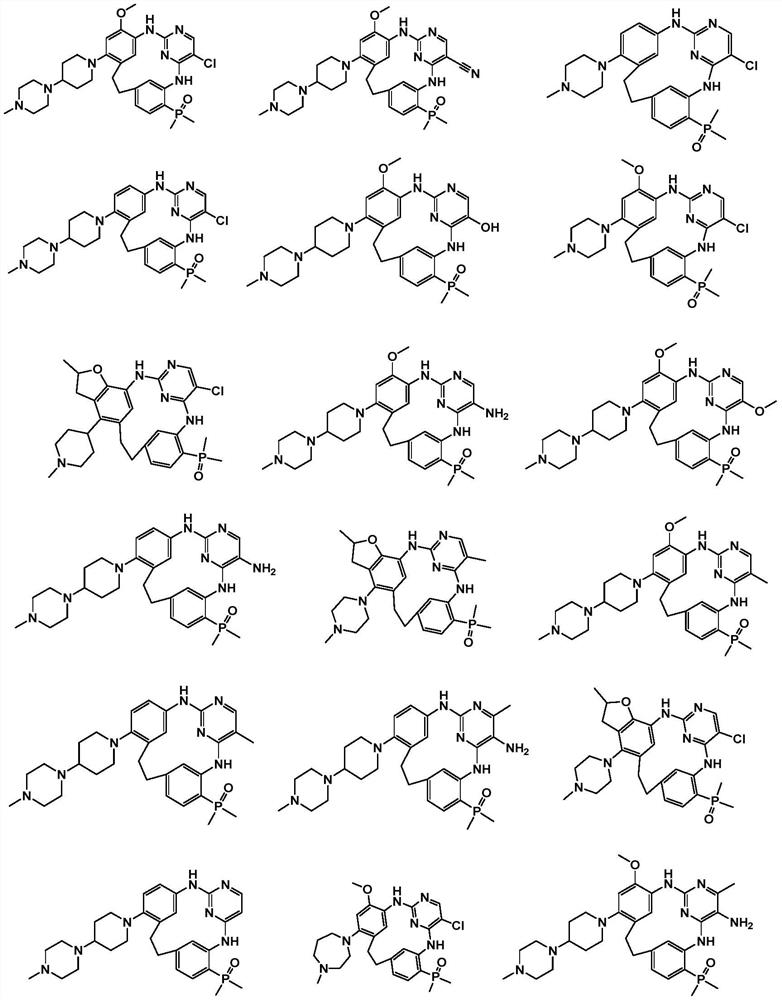
Phosphine-containing macrocyclic compound, and preparation method and application thereof
A technology of macrocyclic compounds and compounds, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, medical preparations containing active ingredients, etc., can solve problems such as treatment failure and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088]
[0089] 10-Dimethylphosphinyl-6-chloro-17-(4-(4-methylpiperazin-1-yl)-piperidin-1-yl)-19-methoxy-2,4,8 ,22-Azatetracyclo-[14.3.1.1 3,7 .1 9,13 ]22cyclo-1(20),3(22),4,6,9(21),10,12,16,18-nonene; MS (ESI) m / z 610.27 [M+H]+.
[0090]
[0091] Step 1: Cool concentrated nitric acid (25.9mmol) to 0°C, slowly add compound 1 (1g, 4.31mmol), continue to stir for 4h, pour the reaction solution into ice water, filter the solid, wash with water, and use di Phosphorus was dried and the crude product was recrystallized (0.45 g, 37%), LC / MS: 278.
[0092] Step 2: Dissolve methyltriphenylphosphine bromide (7.22mmol) in 10ml THF, cool down to -78°C, slowly add n-butyllithium (5.78mmol), and react at room temperature for 30min. The temperature of the reaction solution was lowered to -78°C, compound 2 (3.61 mmol) was dissolved in 4 ml of THF, the above reaction solution was slowly added dropwise, and the reaction was continued at room temperature. TLC monitoring. After the react...
Embodiment 2
[0102]
[0103] 19-methoxy-10-dimethylphosphinyl-6-chloro-17-(4-methylpiperazin-1-yl)-2,4,8,22-azatetracyclo-[14.3. 1.1 3,7 .1 9,13 ]22 cyclo-1(20),3(22),4,6,9(21),10,12,16,18-nonene.
[0104] The preparation of the compound of Example 2 refers to step 1 to step 10 in Example 1, wherein 1-methyl-4-(4-piperidinyl) piperazine in step 6 is replaced by 1-methylpiperazine of equimolar concentration . MS (ESI) m / z 527.20 [M+H]+.
Embodiment 3
[0106]
[0107] 10-Dimethylphosphinyl-6-chloro-17-(4-(4-methylpiperazin-1-yl)-piperidin-1-yl)-2,4,8,22-azatetra ring-[14.3.1.1 3,7 .1 9,13 ]22 cyclo-1(20),3(22),4,6,9(21),10,12,16,18-nonene.
[0108] The preparation of the compound of Example 3 refers to steps 1 to 10 in Example 1, wherein the 1-bromo-2-fluoro-4-methoxy-5-nitrobenzene in step 6 is composed of 2-bromo- 1-fluoro-4-nitrobenzene instead. MS (ESI) m / z 580.26 [M+H]+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com