Antibacterial composition against drug-resistant bacteria or pro-inflammatory bacteria
An antibacterial composition and an inflammation-inducing technology, which can be used in antibacterial drugs, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as intestinal colonization of unconfirmed bacterial strains, and achieve the effect of improving or preventing diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
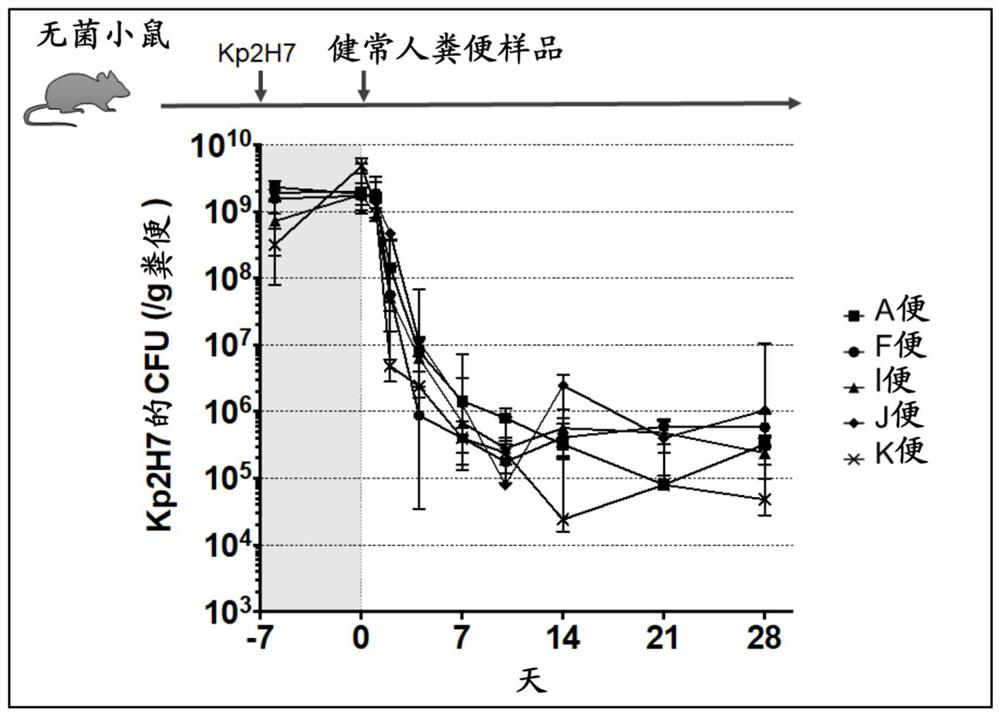
[0184] Such as figure 1 As shown in the upper part of , Klebsiella 2H7 strain (Kp2H7) was administered to germ-free mice, and a stool sample of a healthy volunteer was administered one week later.
[0185] Specifically, for germ-free mice, C57BL / 6N (Japan CLEA Co., Ltd.) 4 to 8-week-old mice were kept in plastic isolators (aseptic isolators) (manufactured by ICM Co., Ltd.; ICM-1B). , fed with free drinking water for more than 1 week, and used after acclimatization to the environment. The age at the beginning of the experiment ranged from 8 to 14 weeks. The same applies to other examples in this specification.
[0186] Put the bacterial liquid of Klebsiella into LB liquid medium and culture it overnight at 37°C, adjust the OD value to 1.2 (equivalent to 1*10^9CFU / mL), and use a probe to inject 200μL / ( Bacterial solution equivalent to 2*10^8CFU / monkey) was administered to the stomach of mice.
[0187] Regarding stool samples, stools provided by healthy Japanese volunteers (#...
Embodiment 2
[0191] (Embodiment 2) Bacteria is isolated from healthy volunteer's feces
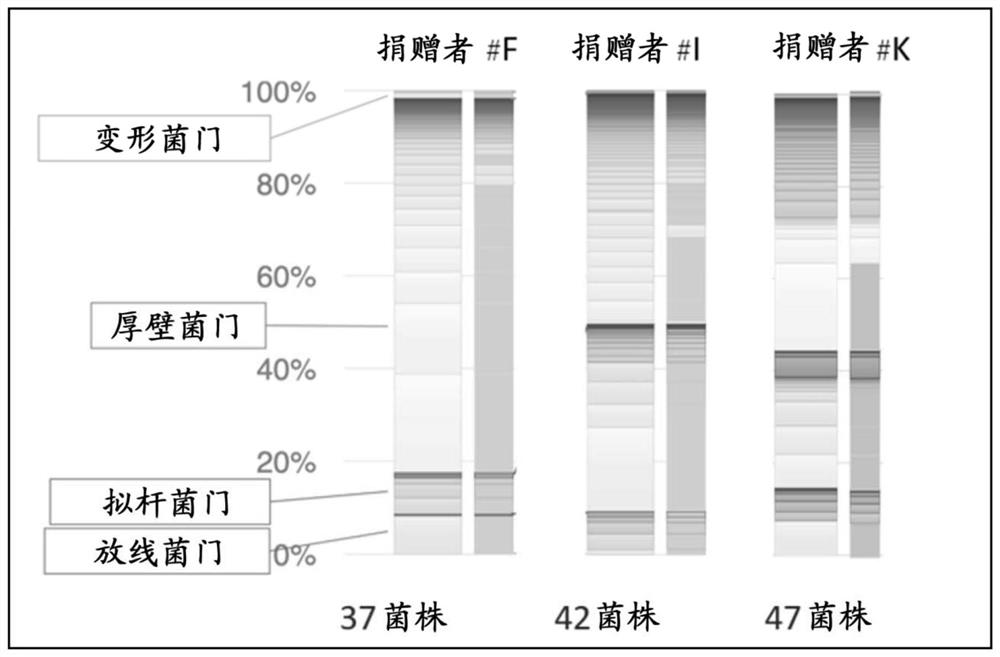
[0192] The frozen stool samples derived from the stools (F stools, K stools, and I stools) from healthy people F, K, and I prepared in Example 1 were thawed at room temperature, diluted with PBS, and placed in EG medium. , modified GAM agar medium (Nissui Pharmaceutical Co., Ltd.; 05426), REINFORCED CLOSTRIDIAL AGAR (RCM AGAR) (Thermo Fisher Scientific Inc; CM0151) or Schaedler blood medium (manufactured by Wako; 517-45805) In 37℃, 10% CO 2 The culture was carried out under anaerobic environment, and the colonies formed were isolated. 37 strains were isolated from F stool, 42 strains were isolated from I stool, and 47 strains were isolated from K stool. Afterwards, K was isolated again, and finally 68 strains were isolated from K.
[0193] The isolated bacteria were analyzed by 16SrDNA using the Sanger method to analyze the gene sequence and estimate the bacteria. Sequencing analysis was performed ...
Embodiment 3
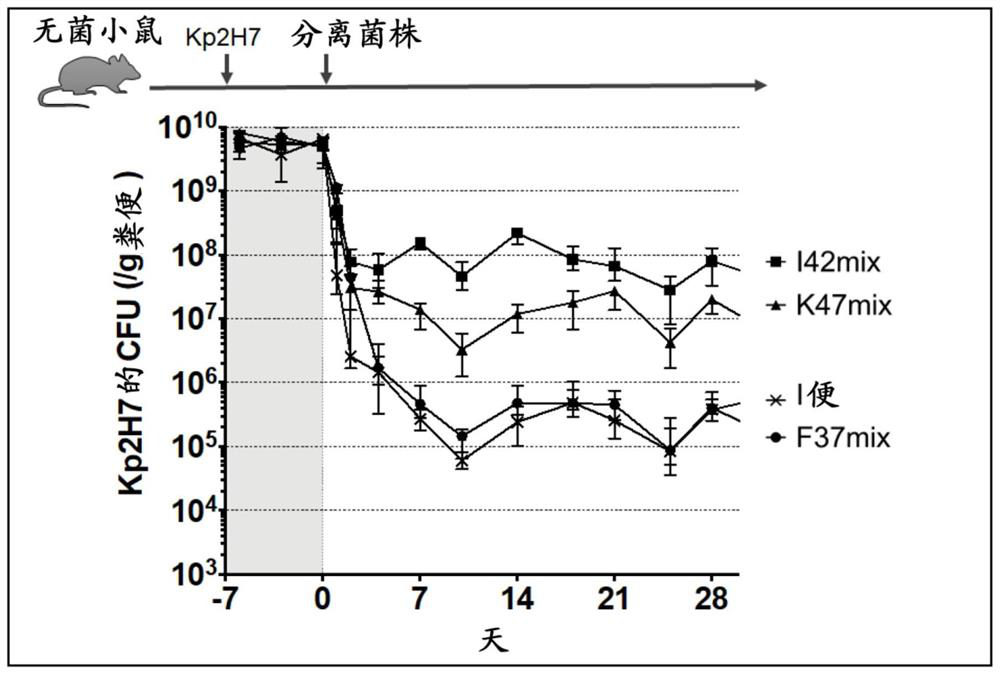
[0200] Such as image 3 As shown in the upper part, Kp2H7 was administered to germ-free mice, and the mixed isolates (37 strains (F37mix) derived from F stool, 42 strains (I42mix) derived from I stool, and 42 strains (I42mix) derived from K Just 47 strains (K47mix)) or 1 just.
[0201] The isolated bacteria use mGAM liquid medium, EG medium or CM0149 medium, cultured in an anaerobic chamber at 37°C for 24-48 hours and mixed. The mixture was concentrated 5 times, and 200 μL / monkey (total bacteria amount equivalent to 1*10^9 CFU / bird) of the bacterial solution was administered into the stomach using a probe. The administration of the mixed isolated strains in the following examples was also carried out in the same manner.
[0202] The result is as image 3 As shown in the lower part (graph), it is shown that the 37 strains derived from F feces are related to the elimination ability of Klebsiella from the intestinal tract of mice, and have the same activity as I feces.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com