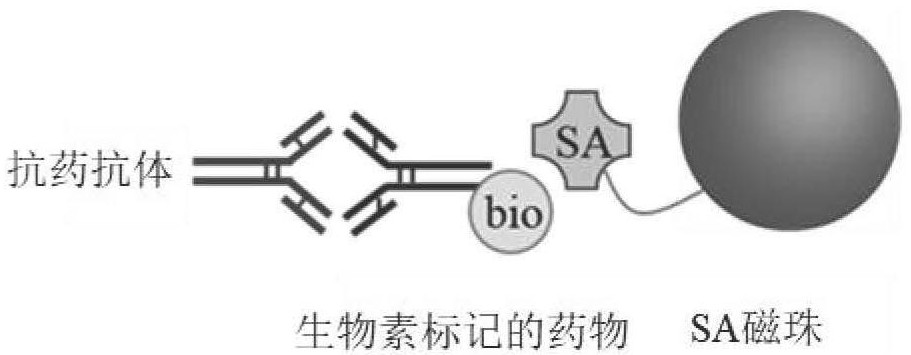
Operation method for improving drug tolerance in drug-resistant antibody analysis
An operation method and drug technology, which can be used in analytical materials, preparation of test samples, measurement devices, etc., can solve problems such as poor drug tolerance, low concentration, and insignificant, to reduce complexity, avoid recombination, The effect of increased drug tolerance concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]Example 1 is a monoclonal antibody drug, and Example 2 is a bispecific antibody drug.
[0046] Embodiment one
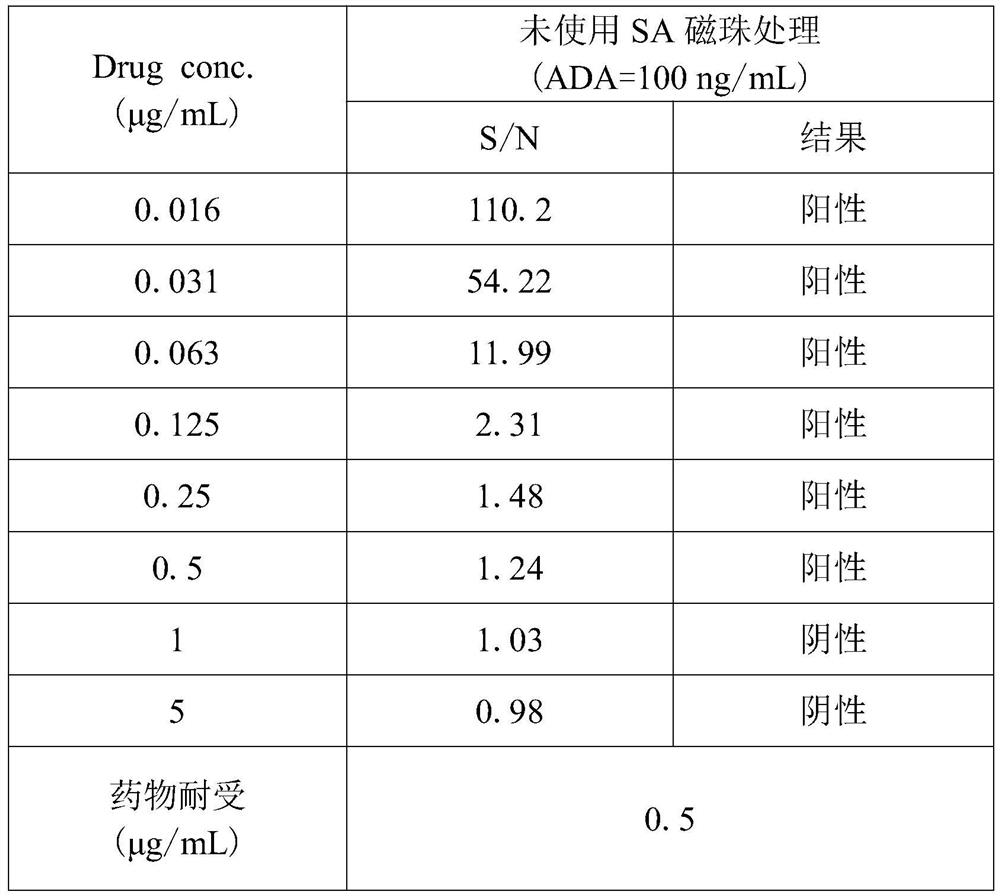
[0047] The SCP is 1.04, the minimum dilution ratio (MRD) is 20, and the ADA concentration is 100ng / mL, which means that the ADA concentration is 100ng / mL, and the maximum drug tolerance corresponding to the enrichment process without SA magnetic beads is 0.5μg / mL , that is, when the maximum drug concentration is 0.5 μg / mL, ADA concentration of 100 ng / mL or above is positive.
[0048] Table 1: Drug tolerance without SA magnetic beads treatment
[0049]
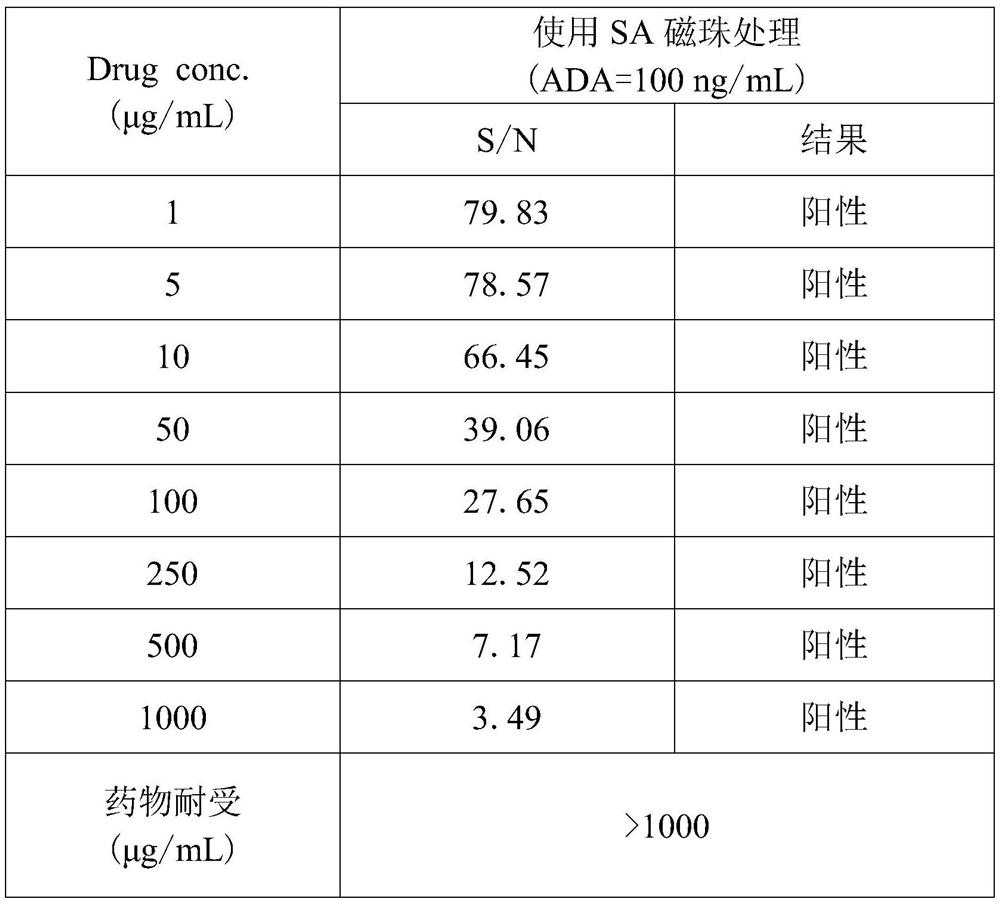
[0050] Under the same conditions above, adding the SA magnetic bead enrichment treatment step, the corresponding maximum drug tolerance is 1000 μg / mL, and the corresponding S / N value is still much higher than the SCP value (1.04), that is, the maximum drug concentration is When 1000μg / mL, ADA concentration of 100ng / mL was positive.
[0051] Table 2: Drug tolerance after treatment with SA magnetic beads
...
Embodiment 2
[0055] The SCP is 1.30, the minimum dilution ratio (MRD) is 5, and the ADA concentration is 100ng / mL, which means that the ADA concentration is 100ng / mL, and the maximum drug tolerance corresponding to the pre-treatment without magnetic bead enrichment is 0.031μg / mL mL, that is, when the maximum drug concentration is 0.031 μg / mL, the ADA concentration is 100 ng / mL, which is positive.
[0056] Table 3: Drug tolerance without SA magnetic beads treatment
[0057]
[0058] Under the same project conditions above, adding the pre-treatment steps of magnetic beads enrichment, the corresponding maximum drug tolerance is 1000 μg / mL, and the corresponding S / N value is still much higher than the SCP value (1.30), that is, the drug concentration is the largest When the concentration of ADA is 100ng / mL, it is positive.
[0059] Table 4: Drug resistance treated with SA magnetic beads
[0060]
[0061] By comparing the data in Table 3 and Table 4, it can be concluded that after using...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com