Process for producing a crosslinked cellulose ether
A technology of cellulose ether and cellulose, applied in the field of producing cross-linked cellulose ether, can solve problems such as excessive cross-linking affecting the process, and achieve the effects of excellent effectiveness, low water solubility and reduced dosage of cross-linking agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0076] The following examples are provided to illustrate the invention in additional detail, but should not be construed as limiting the scope of the claims. All parts and percentages are by weight unless otherwise indicated.
[0077] Various terms and nomenclature used in Inventive Examples (Inv.Ex.) and Comparative Examples (Comp.Ex.) are explained as follows:
[0078] "HEMC" stands for hydroxyethylmethylcellulose.
[0079] "AGU" stands for anhydroglucose unit of cellulose ether.
[0080] "LVN" stands for the Intrinsic Viscosity Number of pulp as measured according to the procedure described in ISO 5351 (2010).
[0081] The various raw materials or ingredients used in the examples are explained below:
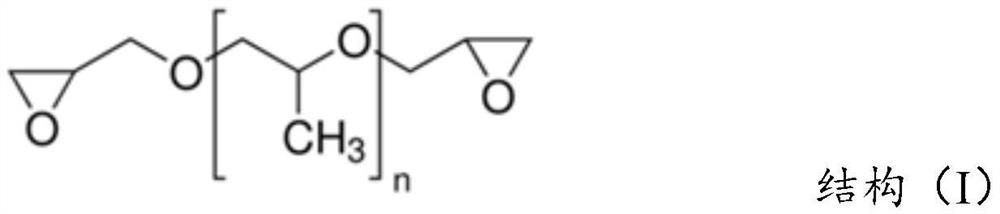
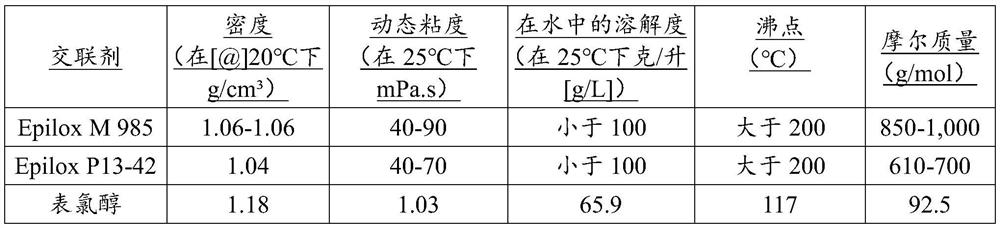
[0082] Epilox M 985 is a poly(propylene glycol) diglycidyl ether commercially available from Leuna Harze.
[0083] Examples of diglycidyl ether-based crosslinking compounds useful in the process of the invention are described in Eugene W. Jones, Crosslinking of Cotton Cel...
example 1 and 2
[0089] Inventive Examples 1 and 2 and Comparative Examples A-C
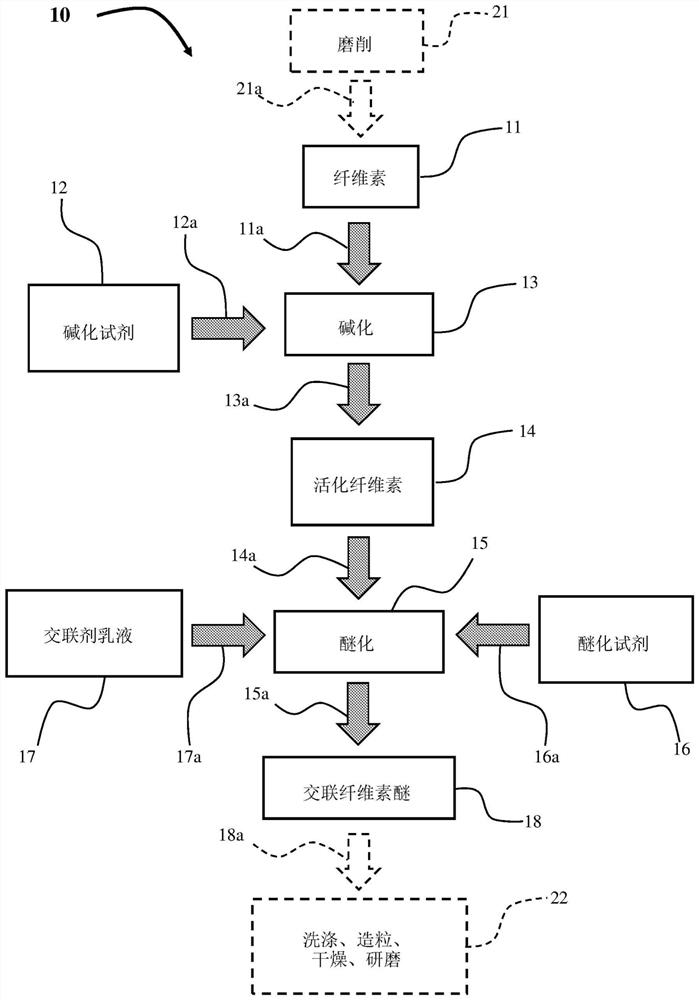
[0090] Typically, HEMC is produced on the principle of Williamson ether synthesis. After activating the ground cellulose with 50% caustic soda, alkalized cellulose is produced. It was then etherified with MCl and EO. For example, a useful guide to this method is found in R. Donges, "Non-Ionic cellulose ethers", British Polymer Journal vol. 23, pp. 315- 326 pages (1990). The crosslinking compounds used in the method of the invention are based on diglycidyl ethers; and a summary of the properties of the crosslinkers used in the examples is described in Table II.
[0091] The intrinsic viscosity number (LVN) of pulp is measured according to the procedure described in ISO 5351 (2010). Ground cellulose fluff (400 mol; LVN greater than or equal to (≧) 1,450 milliliters per gram [mL / g]) was added to a 1,000 liter (L) autoclave (reactor).
[0092] use N 2 After purging the autoclave three times, the autoclave was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com