Method for predicting boiling point of polycyclic aromatic hydrocarbon compound based on molecular energy data
A polycyclic aromatic hydrocarbon and data prediction technology, applied in nuclear methods, chemical property prediction, complex mathematical operations, etc., can solve problems such as poor interpretability of results and many parameters, and achieve the effect of avoiding experiments, simple operation process, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
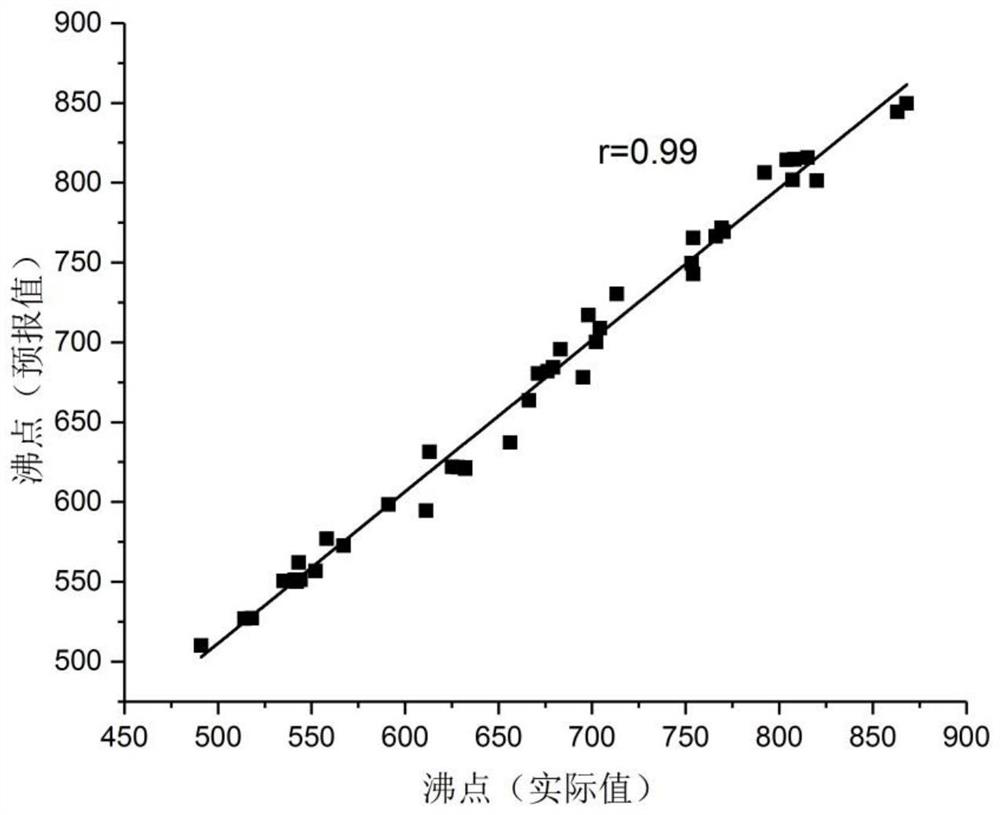
[0027] Embodiment 1: Based on 4 quantum chemical structure energy descriptors of 50 polycyclic aromatic hydrocarbons, the support vector machine regression structure-activity relationship prediction model of the boiling point of polycyclic aromatic hydrocarbons established, the modeling results are as follows figure 1 shown.
[0028] The support vector machine regression algorithm was used to perform regression modeling on 50 polycyclic aromatic hydrocarbon sample data, and a quantitative prediction model for the boiling point of polycyclic aromatic hydrocarbons was established. The correlation coefficient between the predicted value of the model and the true value in the literature was 0.99.
Embodiment 2
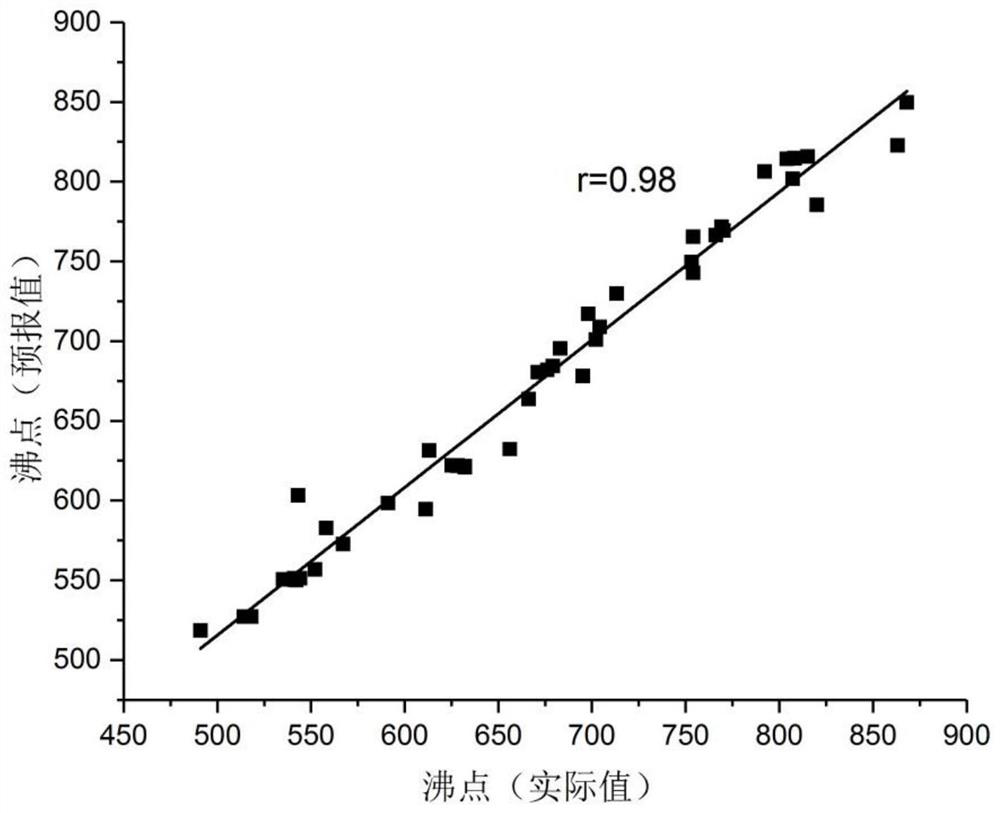
[0029] Embodiment 2: Based on 4 quantum chemical structure energy descriptors of 50 polycyclic aromatic hydrocarbons, the support vector machine regression structure-activity relationship prediction model of the boiling point of polycyclic aromatic hydrocarbons established, the internal cross-validation results of the model leave-one-out method are as follows figure 2 shown.
[0030] Using the support vector machine regression algorithm to perform regression modeling on 50 polycyclic aromatic hydrocarbon sample data, a quantitative prediction model for the boiling point of polycyclic aromatic hydrocarbons was established. The internal cross-validation results of the model showed that the correlation coefficient between the predicted value of the model and the true value in the literature was 0.98.
Embodiment 3
[0031] Example 3: Table 3 shows the newly collected 4 polycyclic aromatic hydrocarbon compounds, their 4 quantum chemical structure energy descriptors, mapping transformation data, and their boiling point prediction results.
[0032] molecular EHOMO ELUMO ΔE ETot Y 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com