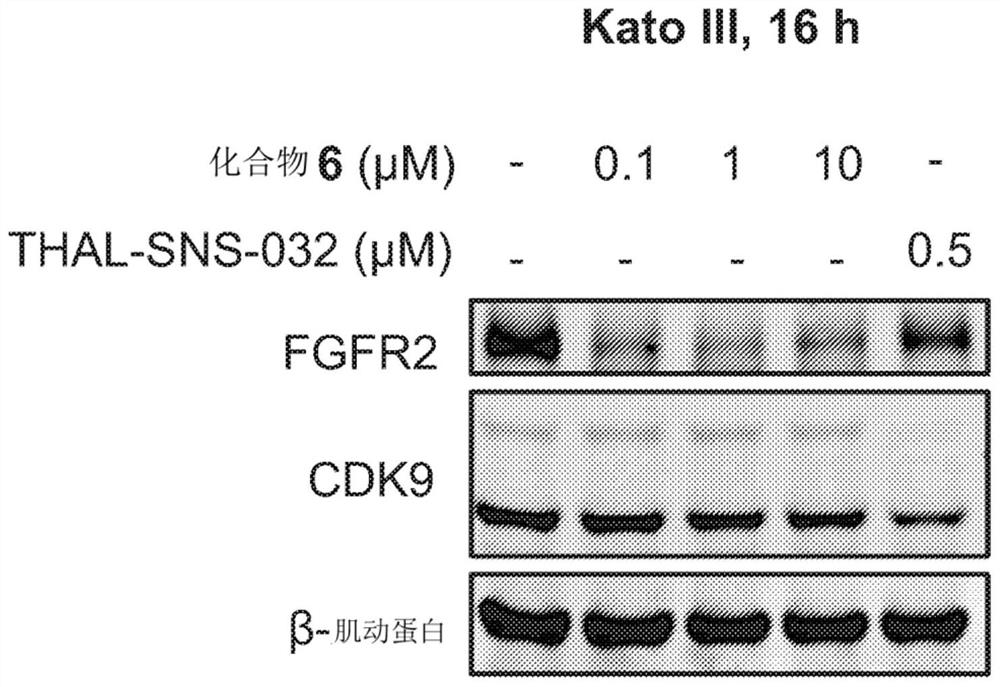
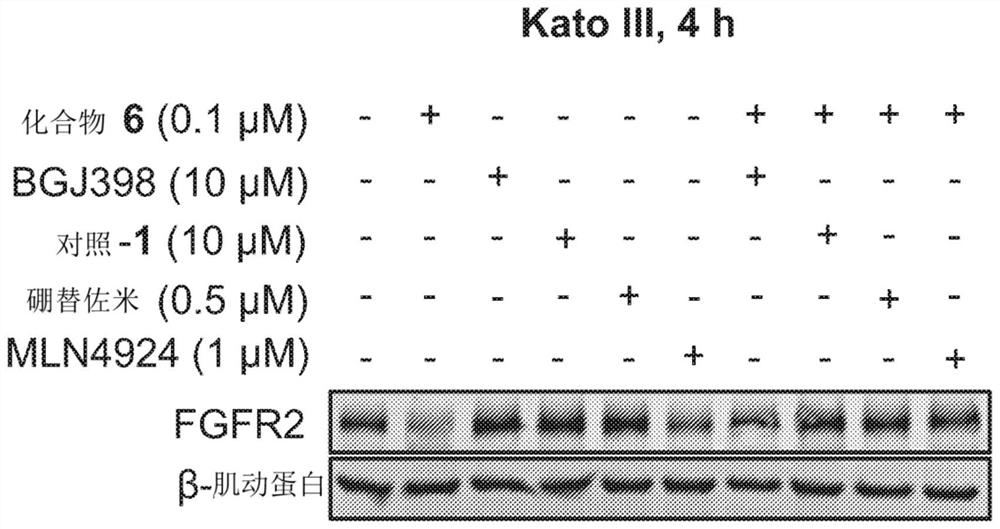
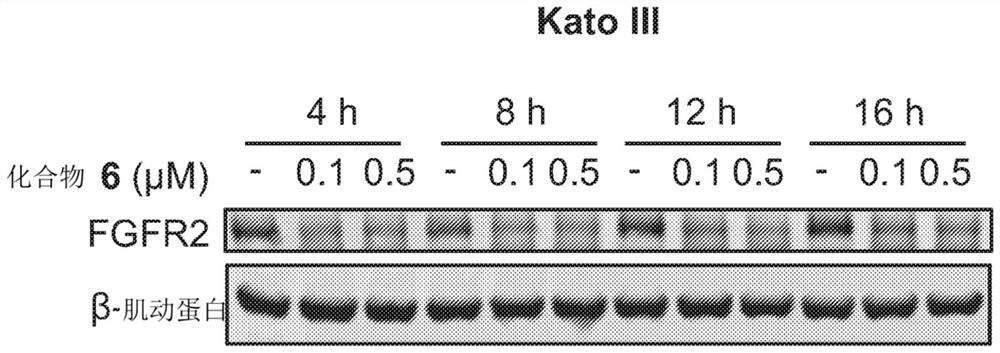
Degraders of fibroblast growth factor receptor 2 (FGFR2)
A technology for growth factor receptors and fibroblasts, applied in the field of degradation agents of fibroblast growth factor receptor 2 (FGFR2), which can solve the problems of low survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] Example 1: Synthesis of Thalidomide-Based Intermediates.
[0247]
[0248] tert-Butyl 2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetate
[0249] tert-Butyl bromoacetate (199 mg, 1.02 mmol) was added to 2-(2,6-dioxopiperidin-3-yl)-4-hydroxyisoindoline-1,3-dione ( 200mg, 0.) 3mmol) and K 2 CO 3 (304 mg, 2.20 mmol) in a mixture in 3 mL of DMF. The mixture was stirred overnight, then quenched with water. The aqueous mixture was then extracted with 3 × 5 mL ethyl acetate, washed with brine, washed with Na 2 SO 4Dried and concentrated. Purification by silica gel chromatography provided tert-butyl 2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetate (245 mg, 0.63 mmol, 86%) as a white crystalline solid. LC / MSm / z calculated as [M+2H–tBu] + 333.1, measured 333.1.
[0250]
[0251] 2-((2-(2,6-Dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl)oxy)acetic acid
[0252] tert-butyl 2-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-4-yl...
Embodiment 2
[0263] Example 2: 2-(4-{4-[(6-{[(2,6-dichloro-3,5-dimethoxyphenyl)carbamoyl](methyl)amino}pyrimidine-4 -yl)amino]phenyl}piperazin-1-yl)-N-{2-[2-(2-{[3-(2,6-dioxypiperidin-3-yl)-2,4- Dioxo-3-azatricyclo[).3.1.0 5 , 13 Synthesis of ]trideca-1(12),5,),9(13),10-penten-)-yl]oxy}ethoxy)ethoxy]ethyl}acetamide (1)
[0264]
[0265] tert-butyl 4-(4-((6-chloropyrimidin-4-yl)amino)phenyl)piperazine-1-carboxylate
[0266] 4-(4-Aminophenyl)piperazine-1-carboxylate tert-butyl ester (2.0 g, .21 mmol) was added to 4 in DIEA (1.88 mL, 10.8 mmol) and isopropanol (15 mL), 6-dichloropyrimidine (1.61 g, 10.8 mmol). The purple solution was then stirred overnight at room temperature. The solvent was evaporated and the residue was purified by silica gel chromatography to give the title compound as a maroon solid (2.) 5 g, ).05 mmol, 98%). LC / MS m / z calculated as [M+H] + 390.16, measured 390.30.
[0267]
[0268] tert-Butyl 4-(4-((6-(methylamino)pyrimidin-4-yl)amino)phenyl)piperazine-1-c...
Embodiment 3
[0282] Example 3: 2-(4-{4-[(6-{[(2,6-dichloro-3,5-dimethoxyphenyl)carbamoyl](methyl)amino}pyrimidine-4 -yl)amino]phenyl}piperazin-1-yl)-N-(3-{[3-(2,6-dioxopiperidin-3-yl)-2,4-dioxo-3-nitrogen Heterotricyclic[).3.1.0 5 , 13 Synthesis of ]trideca-1(12),5,),9(13),10-penten-)-yl]oxy}propyl)acetamide (2)
[0283]
[0284] The synthesis method of bispecific compound 2 is similar to that of bispecific compound 1. LC / MS m / z calculated as [M+H] + 953.28, measured 953.53.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com