Compounds Used to Lower Uric Acid Levels
A uric acid level and compound technology, applied in drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as hepatotoxicity and acute liver failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Synthesis of compound I and its PDE4 inhibitory activity experiment
[0085] For the synthesis method of compound I and its PDE4 inhibitory activity test, please refer to the relevant description in the example of Chinese patent CN111808098B (paragraph [0082] on page 10-paragraph [0114] on page 14 of the specification).
Embodiment 2
[0086] The clinical trial of embodiment 2 compound I
[0087] 2.1 Clinical trial protocol design
[0088]
[0089]
[0090]
[0091]
[0092]
[0093]
[0094] 2.2 Results of clinical trials
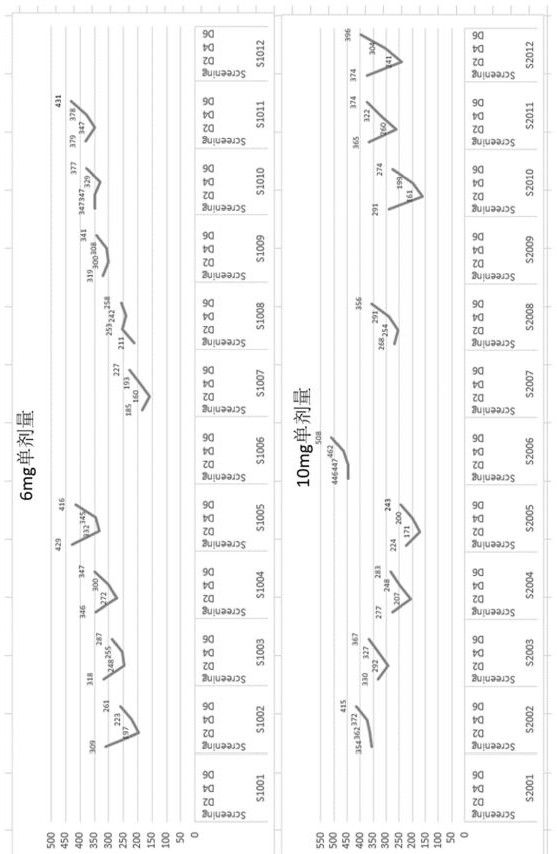
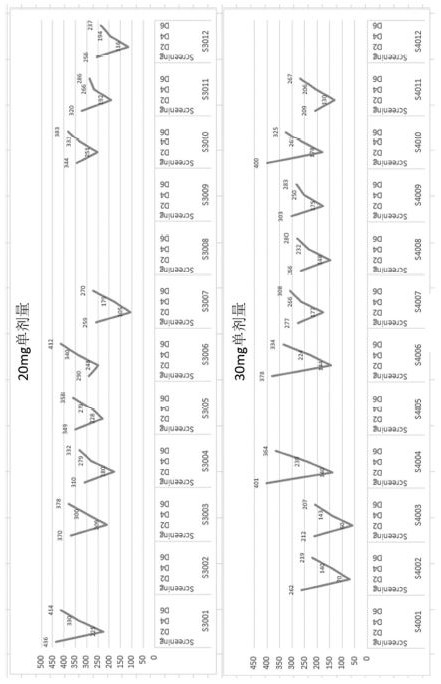
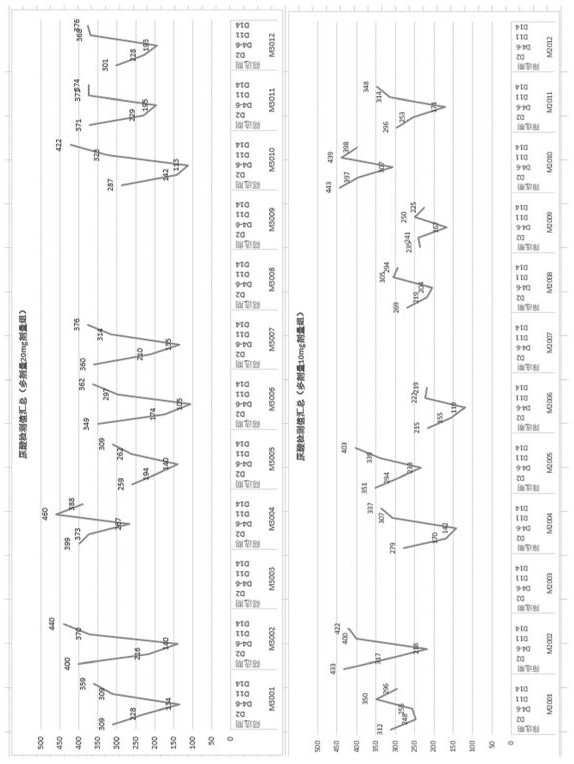
[0095] The uric acid levels of the subjects in the single-dose group and the multi-dose group of Compound I changed as follows: Figure 1A (6 mg single dose and 10 mg single dose), Figure 1B (20 mg single dose and 30 mg single dose), figure 2 (10 mg multiple dose and 20 mg multiple dose), the results are also shown in Table 1.1, Table 1.2, Table 1.3 and Table 2.1, and Table 2.2, Table 2.3 and Table 2.4. Among them, Table 1.1, Table 1.2 and Table 1.3 show the changes in the uric acid level values of the subjects in the single dose group (6 mg, 10 mg, 20 mg and 30 mg) before and after receiving Compound I treatment, Table 2.1, Table 2.2, Table 2.3 and Table 2.4 show the changes in the uric acid level values of the subjects in the multi-dose groups (10 mg and ...
Embodiment 3
[0106] Example 3 Comparison of Compound I with other uric acid-lowering drugs
[0107] According to the clinical trial data of Example 2, based on the results of uric acid changes from baseline after 7 days of continuous administration of compound I at two different doses (10 mg and 20 mg) once a day, the inventors used the PK / PD model for compound I The uric acid-lowering effect of another four different doses (6 mg, 15 mg, 30 mg and 40 mg) administered once a day for 7 days was predicted. For specific results, please refer to image 3. Single-drug and combined use data of Recinard and febuxostat disclosed in the literature (see the literature Jacob Leander, Journal of Pharmacokinetics and Pharmacodynamics https: / / doi.org / 10.1007 / s10928-021-09747-y, and Figure 4 ) compared with the same or lower doses, Compound I showed better uric acid-lowering effects than single drugs of Resinald and febuxostat, and even achieved the same effect as the combination of Resinald and febuxos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com