Orally disintegrating tablets comprising glycopyrrolate and methods for increasing bioavailability
A technology of glycopyrronium bromide and disintegrant, which is applied in the field of orally disintegrating pore glycopyrronium bromide tablets, and can solve problems such as the bioavailability of unspecified compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
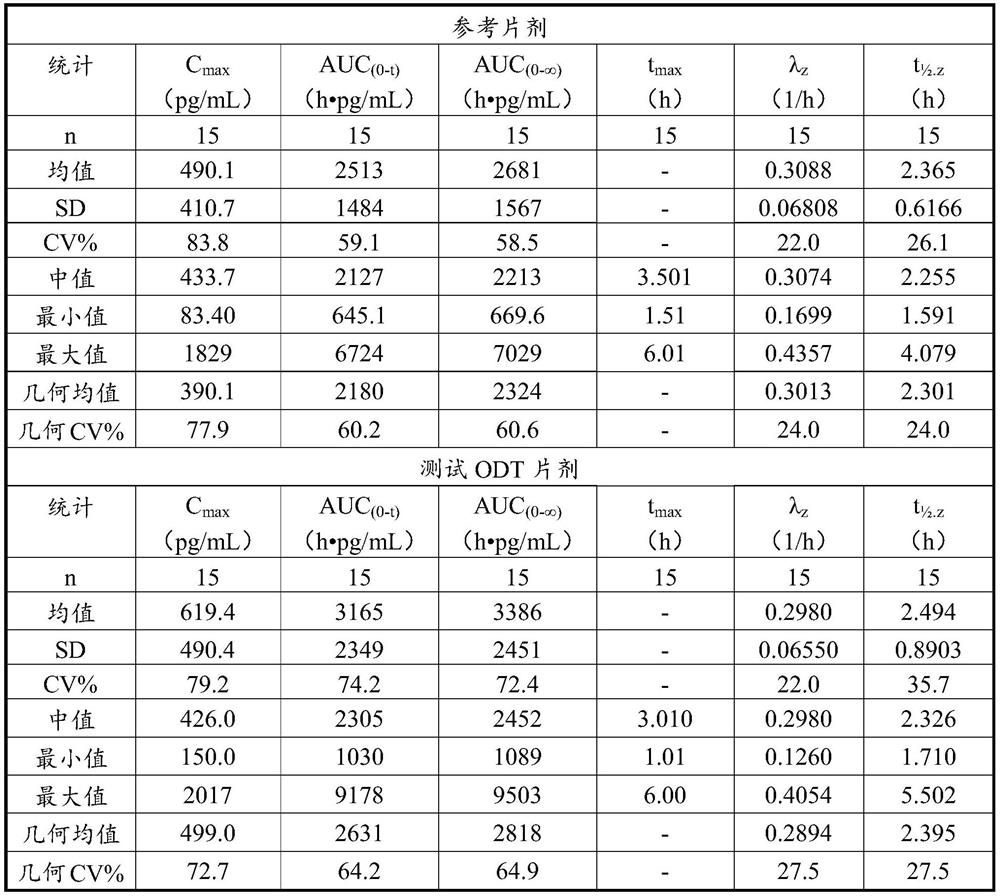
[0063] Example 1 - Comparative Pharmacokinetics and Bioavailability Analysis
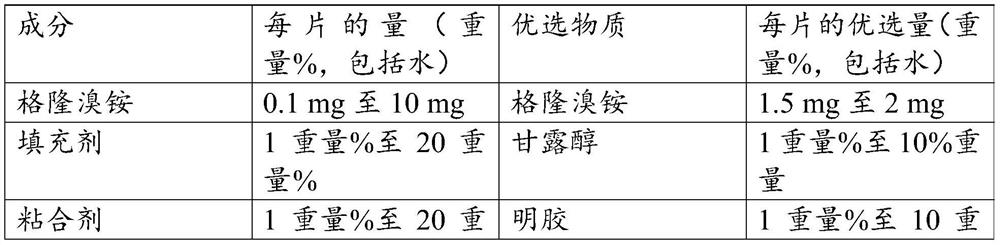
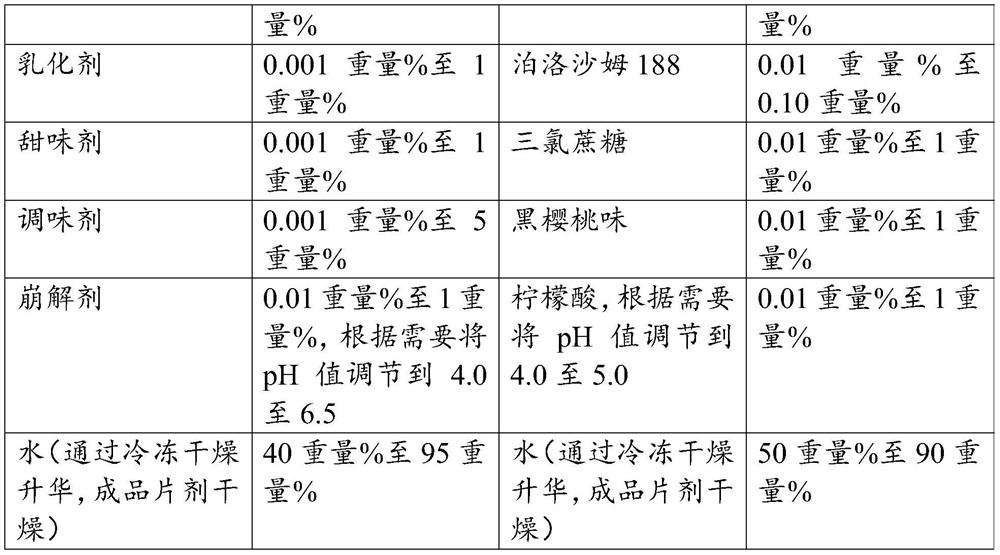
[0064] Oral tablets containing 2 mg glycopyrronium bromide were prepared by mixing a liquid formulation containing glycopyrronium bromide, mannitol, gelatin, poloxamer 188, sucralose, flavoring, and citric acid with water as shown in Table 1. Disintegrate pogoronium bromide tablets. Adjust the pH of the liquid formulation to 4.0-5.0. The suspension is then freeze-dried and tablets are produced. The resulting tablets were used as test oral bengeglurronium bromide tablets (Test ODT) in the pharmacokinetic and bioavailability analyses, reported below.
[0065] Pharmacokinetic and bioavailability analyzes were performed on oral bengyrronium bromide tablets (test ODT) containing 2 mg glycopyrronium bromide. The analysis was performed in comparison to a reference oral tablet from the company Par Pharmaceutical, also containing 2 mg glycopyrronium bromide, referred to as the reference tablet.
[0066]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com