Tandem epitope polypeptide vaccine of novel coronavirus and application thereof
A coronavirus and epitope technology, applied in the direction of viral peptides, antiviral immunoglobulins, viruses, etc., can solve the problems of low response rate of the crowd, low immunogenicity, difficulty in overcoming virus immune escape, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Example 1 T / B cell epitope screening and tandem epitope polypeptide design based on the sequence and structure analysis of S protein RBD
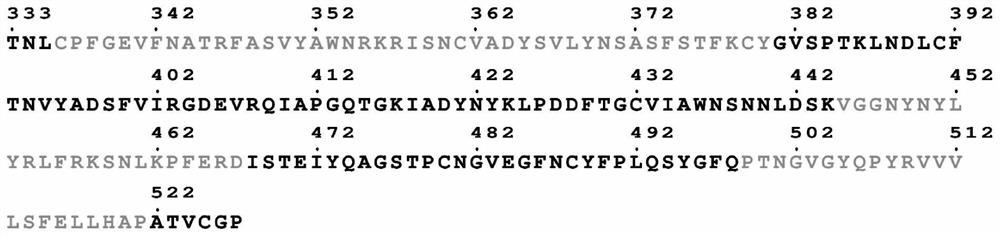
[0168] By analyzing the sequence and structure of the S protein RBD of SARS-CoV-2, the present inventors predicted and analyzed the CD4+T / CD8+T cell epitope, linear / conformation B Cellular epitopes, structural features and key sites of interaction. After comprehensive analysis of the results, suitable T / B cell antigen epitopes were screened and determined, and these epitopes were concatenated with the universal Th epitope PADRE sequence, wherein the epitopes were connected by four glycines to ensure mutual non-interference.
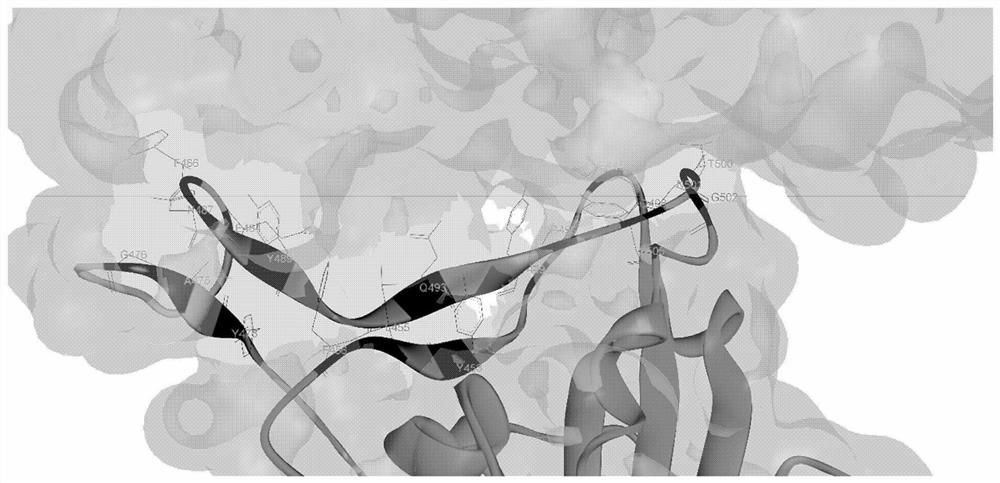
[0169] Specifically, the inventors took the RBD region interacting with human ACE2 in the SARS-CoV-2 virus S protein as the analysis object, and determined the key sites in the RBD interacting with ACE2, such as figure 1 shown.
[0170] The present inventor also used Allele Frequency Net Database statistical a...
Embodiment 2
[0178] The preparation of embodiment 2 polypeptide
[0179] In this example, a fully automatic solid-phase peptide synthesizer was used to prepare the peptide LP2.
Embodiment 3
[0180] Example 3 The immune effect of LP2 polypeptide vaccine
[0181] In this example, the polypeptide LP2 prepared in Example 2 was used to immunize cynomolgus monkeys, and the immune effect of LP2 was evaluated.
[0182] Mix the polypeptide LP2 with an adjuvant (such as TiterMax) to prepare an immune preparation, immunize cynomolgus monkeys by subcutaneous multi-point injection, and 14 days after the second immunization, use the Bridging-ELISA method to measure the antibody titer, and measure the antiserum Blocking capacity of RBD binding to ACE2.
[0183]The neutralizing antibody was detected by the competitive ELISA method, and the specific determination method was as follows: 10 μg / mL ACE2 was coated overnight on the microtiter plate, and it was blocked before use. Dilute the anti-peptide serum (1:128, 1:64, 1:32, 1:16, 1:8 and 1:4) with sample dilution buffer to different degrees, and then mix the diluted antiserum with 12 μg / mL of Bio-RBD was incubated at 37°C for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com