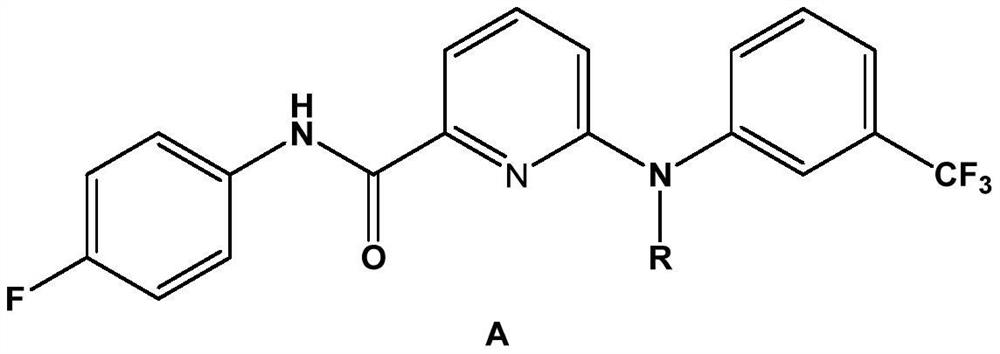
Picolinafen derivative as well as preparation method and application thereof
A technology of flufenamid and its derivatives, which is applied in the field of flufenamid derivatives and its preparation, can solve the problems of no reports and achieve good herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
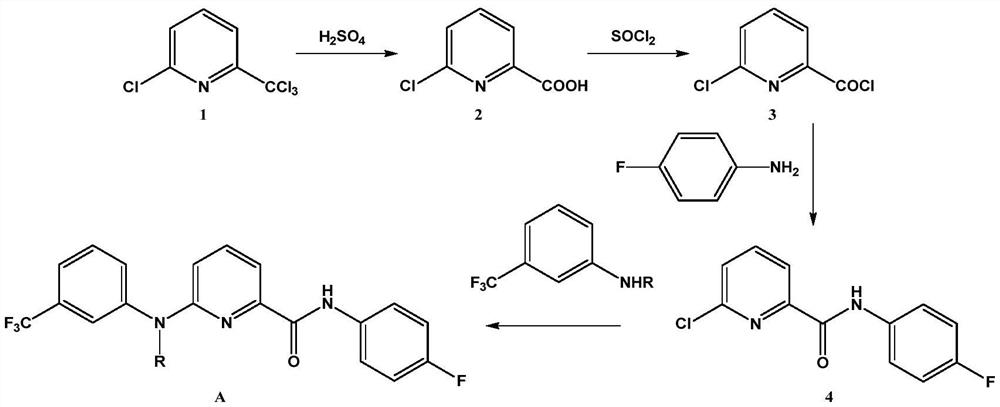
[0026] Embodiment one, the preparation of compound a
[0027] 1. Preparation of Intermediate 2
[0028]
[0029] 2-Chloro-6-trichloromethylpyridine (230.9 g, 1.0 mol) and 98% concentrated sulfuric acid (120 g, 1.2 mol) were successively added into a round bottom flask, and the reaction was stirred at 120° C. for 6 h. After the reaction, the temperature was lowered to 60°C, and 27% ammonia water (172.8ml, 1.2mol) was slowly added dropwise. After cooling to room temperature, suction filtration under reduced pressure gave 146.8 g of a white solid (Intermediate 2), with a yield of 93.5%.
[0030] 2. Preparation of Intermediate 4
[0031]
[0032] In a round-bottomed flask, 2-chloro-6-carboxypyridine (157.0 g, 1.0 mol), thionyl chloride (142.8 g, 1.2 mol), and 300 ml of toluene were sequentially added, and the mixture was refluxed for 5 h. After cooling down to room temperature, p-fluoroaniline (111.1 g, 1.0 mol) was added, and triethylamine (121.5 g, 1.2 mol) was added dr...
Embodiment 2
[0036] Embodiment two, the preparation of compound b
[0037] Except for using N-methyl-m-trifluoromethylaniline instead of m-trifluoromethylaniline, it was synthesized by the same method as that of compound a, and the total yield was 50.6%. The structural formula is as follows:
[0038]
[0039] ESI MS:390.2[M+H] +1 , 1 H-NMR (400MHz, DMSO) δ9.22(s, 1H), 8.02(d, 1H, J=7.5Hz), 7.88(d, 1H, J=7.5Hz), 7.69-7.51(m, 3H), 7.33 (m, 2H), 7.27 (s, 1H), 7.13 (m, 2H), 6.93 (m, 1H), 2.13 (s, 3H).
Embodiment 3
[0040] Embodiment three, the preparation of compound c
[0041] Except for using N-ethyl m-trifluoromethylaniline instead of m-trifluoromethylaniline, it was synthesized by the same method as that of compound a, and the total yield was 51.2%. The structural formula is as follows:
[0042]
[0043] ESI MS:404.6[M+H] +1 , 1 H-NMR (400MHz, DMSO) δ9.17(s, 1H), 8.11(d, 1H, J=7.5Hz), 7.93(d, 1H, J=7.5Hz), 7.62-7.43(m, 3H), 7.35 (m, 2H), 7.27 (s, 1H), 7.16 (m, 2H), 6.97 (m, 1H), 2.27 (m, 2H), 1.35 (m, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com