New application of human-derived lactolectin subtype protein
A technology of lactagglutinin and application, which is applied in the field of human-derived lactaglutinin subtype protein, can solve the problems of unsatisfactory effect and other problems, and achieve the effects of reducing blood-brain barrier leakage, small dosage and short effect time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
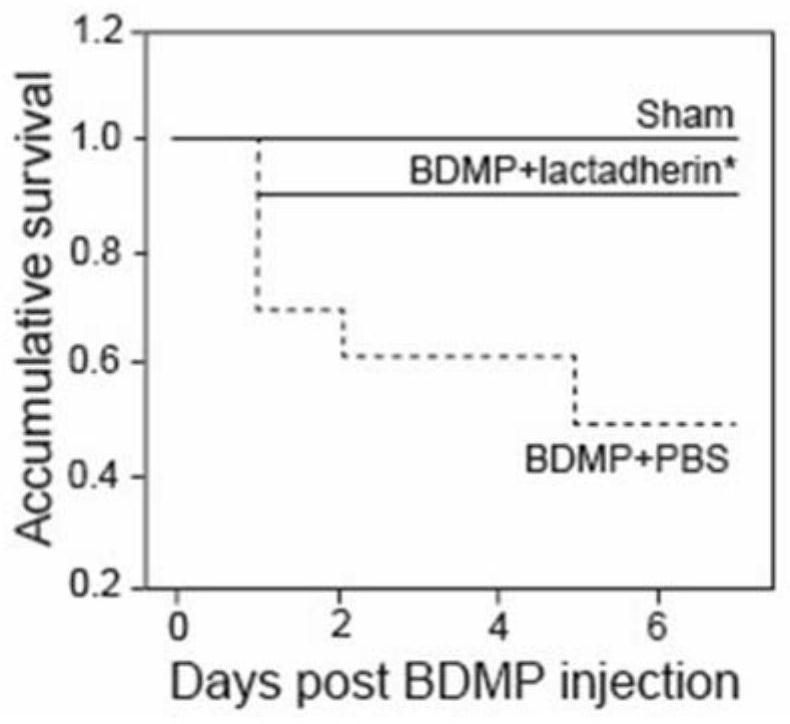
[0085] Lactadherin protein can reduce death and improve prognosis in TBI mice
[0086] 1.1 Objects and methods
[0087] 1.1.1 Experimental mice and instruments
[0088] All experimental mice were from Jackson Laboratory in the United States, and male C57 / BL6 black mice aged 8-20 weeks were used in the experiments, with a body weight ranging from 20 to 26 g. The mice were kept in the Specific Pathogen Free (SPF) grade animal breeding room of BloodWorks Northwest Research Institute. All experimental mice were fed with standard pellet feed and had free access to water. 250Iux, kept by professional animal breeders under the conditions of alternation of day and night.
[0089]
[0090]
[0091] 1.1.2 Experimental reagents
[0092]
[0093]
[0094] 1.1.3 Experimental method
[0095] 1.1.3.1 Preparation of BDMP by Gradient Centrifugation of Mouse Brain Tissue Homogenate
[0096] (1) Take the brain tissue of C57BL / 6 mice frozen in liquid nitrogen, put it in room temp...
Embodiment 2
[0166] Inhibitory effect of Lactadherin protein on mouse TBI-AC
[0167] 2.1 Objects and methods
[0168] 2.1.1 Experimental mice and instruments
[0169] All experimental mice were from Jackson Laboratory in the United States, and male C57 / BL6 black mice aged 8-20 weeks were used in the experiments, with a body weight ranging from 20 to 26 g. The mice were kept in the Specific Pathogen Free (SPF) grade animal breeding room of BloodWorks Northwest Research Institute. All experimental mice were fed with standard pellet feed and had free access to water. 250Iux, kept by professional animal breeders under the conditions of alternation of day and night.
[0170]
[0171]
[0172] 2.1.2 Experimental reagents
[0173]
[0174]
[0175] 2.1.3 Experimental method
[0176] 2.1.3.1 Determination of coagulation time of coagulation factor Xa
[0177] Take the blood of C57 / BL6 mice 1 hour before the TBI blow and 1 hour, 3 hours, and 6 hours after the blow (periorbital oute...
Embodiment 3
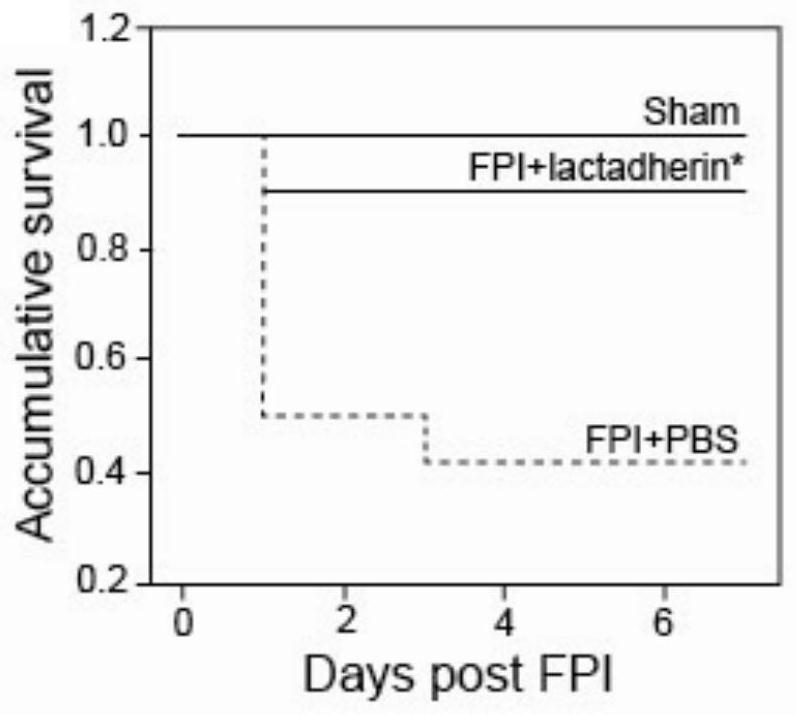
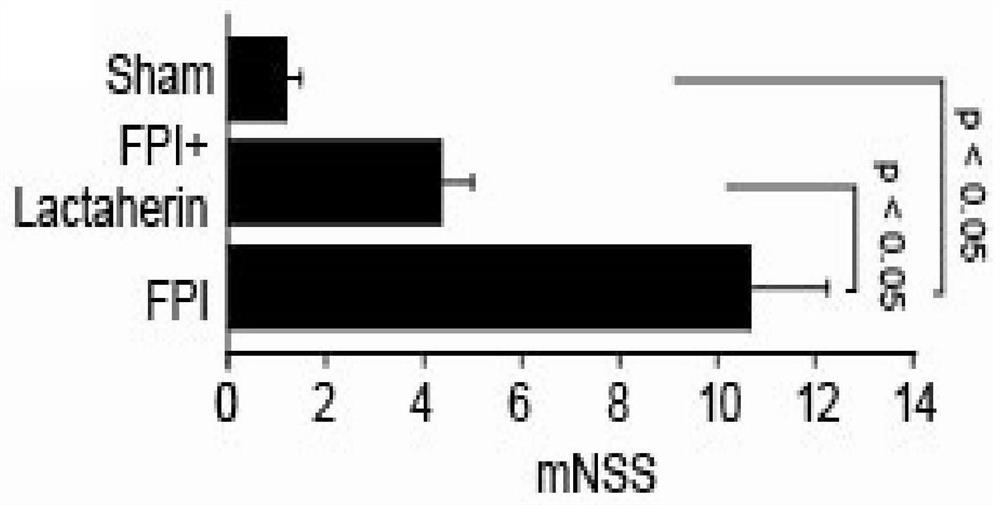
[0246] In vivo experiments to study the mechanism of action of Lactadherin protein
[0247] 3.1 Objects and methods
[0248] 3.1.1 Experimental mice and instruments
[0249] All experimental mice were from Jackson Laboratory in the United States, and male C57 / BL6 black mice aged 8-20 weeks were used in the experiments, with a body weight ranging from 20 to 26 g. The mice were kept in the Specific Pathogen Free (SPF) grade animal breeding room of BloodWorks Northwest Research Institute. All experimental mice were fed with standard pellet feed and had free access to water. 250Iux, kept by professional animal breeders under the conditions of alternation of day and night.
[0250]
[0251]
[0252] 3.1.2 Experimental reagents
[0253]
[0254]
[0255] 3.1.3 Experimental method
[0256] 3.1.3.1 Biotinylated BDMPs
[0257] Use a commercially purchased EZ-Link TM Sulfo-NHS-Biotin kit (ThermoFisher Scientific, Waltham, MA)
[0258] (1) Wash the extracted BDMP once ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com