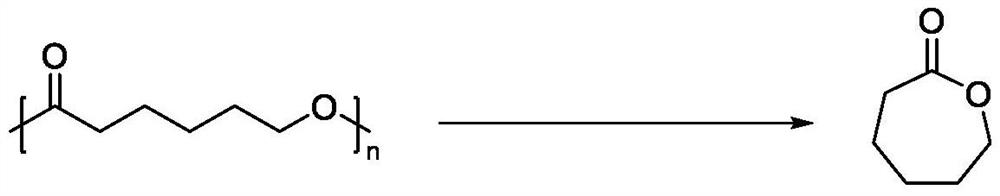
Method for recovering caprolactone from poly(epsilon-caprolactone) waste
A caprolactone polymer, caprolactone technology, applied in the direction of organic chemistry, can solve the problem of high temperature, achieve the effects of good universality, enhanced production efficiency, and economical production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Double (double trimethylsilicide) amine magnesium-involved poly ε-caprolactone depolymerization.
[0027]
[0028] The experiment process includes the following steps:
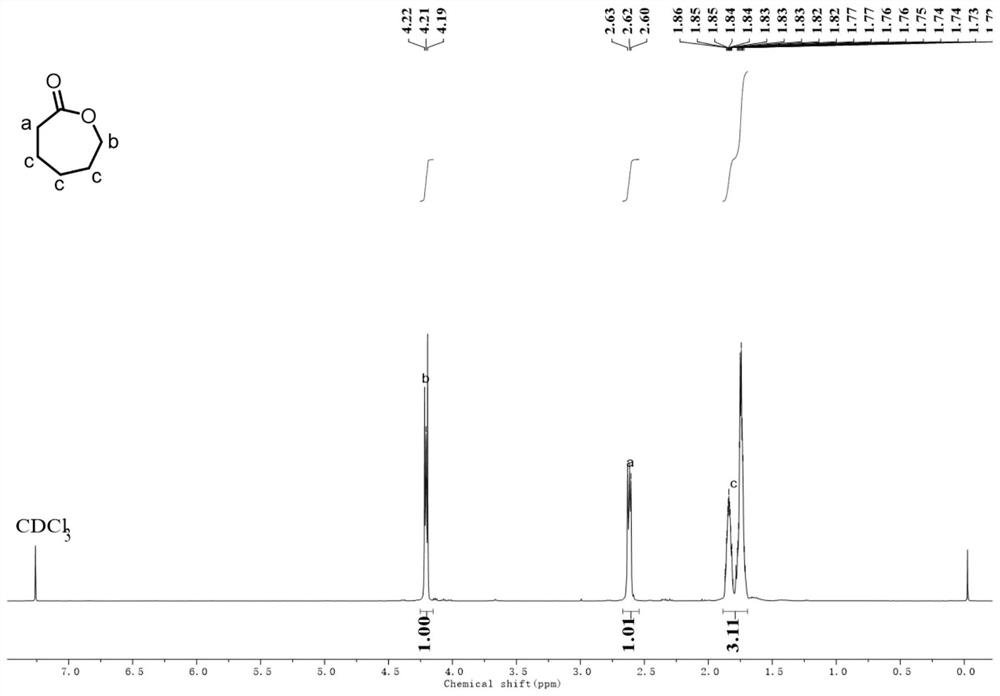
[0029] Poly ε-hexyxer was added (11.4 g, 1000equiv.) Was added to the three flasks, and then bis (double trimethylsilicide-based) amine magnesium phasing reagent (0.138 g, 4equiv.) Was added to the glove box. The temperature was heated to 180 ° C while the decompression (0.3 mbar) was distilled off 7 h to obtain the ethylene derivative product. The monomer hexhane is obtained. The yield was 85%.
Embodiment 2
[0031] Double (double trimethylsilicide) amine magnesium-involved poly ε-caprolactone depolymerization.
[0032] The experiment process includes the following steps:
[0033] Add poly ε-hexyxerry waste (114.0 g, 1000equiv.) In a three flask (114.0 g, 1000equiv.) And then added a bis (double trimethylsilicide) amine magnesium polymerization reagent (1.38 g, 4equiv.). The temperature was heated to 180 ° C while the decompression (0.3 mbar) was distilled 8h to obtain the erythoxide of the polymerized product. The monomer-caprolactone 112 g was obtained. The yield was 98%.
Embodiment 3
[0035] Double (double trimethylsilicide) amine magnesium-involved poly ε-caprolactone depolymerization.
[0036] The experiment process includes the following steps:
[0037] Add poly ε-adiponic waste (11.4 g, 1000equiv.) In three flasks, and then added a double (double trimethylsilicide) amine magnesium polymerization reagent (34.5 mg, 1equiv.) In a glove box. The temperature was heated to 180 ° C while the decimination of the decompression (0.3 mbar) was evaporated. The monomer hexhane is obtained from 9.5 g. The yield was 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com