5-substituted pyridazine-4-amine derivative, preparation method and application
A technology of amine derivatives and pyridazine, which is applied in the field of compound synthesis and application, can solve the problems of pyridazine derivative synthesis and less research on anti-tumor activity, and achieve excellent inhibitory activity, simple preparation method, and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] Concrete preparation method comprises the following steps:
[0048] 1) Under nitrogen protection, 5-bromopyridazin-4-amine N, N-dimethylformamide DMF and water to obtain a mixed solution A;
[0049] 2) Add compound A, inorganic base and palladium catalyst to the mixed solution in step 1), and stir at 80° C. to 120° C. for 1 to 5 hours; obtain mixed solution B;
[0050] 3) Dilute the mixed solution B with water, extract with ethyl acetate, take the organic phase, wash, dry, and concentrate under reduced pressure to obtain the crude product;
[0051] 4) The obtained crude product is separated and purified by column chromatography to obtain the target product.
[0052] Further, in the step 1), the mass volume ratio of 5-bromopyridazin-4-amine to N,N-dimethylformamide is 1000-3000mg:20-50mL; the N,N-dimethylformamide The volume ratio of methyl formamide and water is 1-4:1-10.
[0053] Further, in the step 2), the molar mass ratio of compound A to 5-bromopyridazin-4-ami...
Embodiment 1
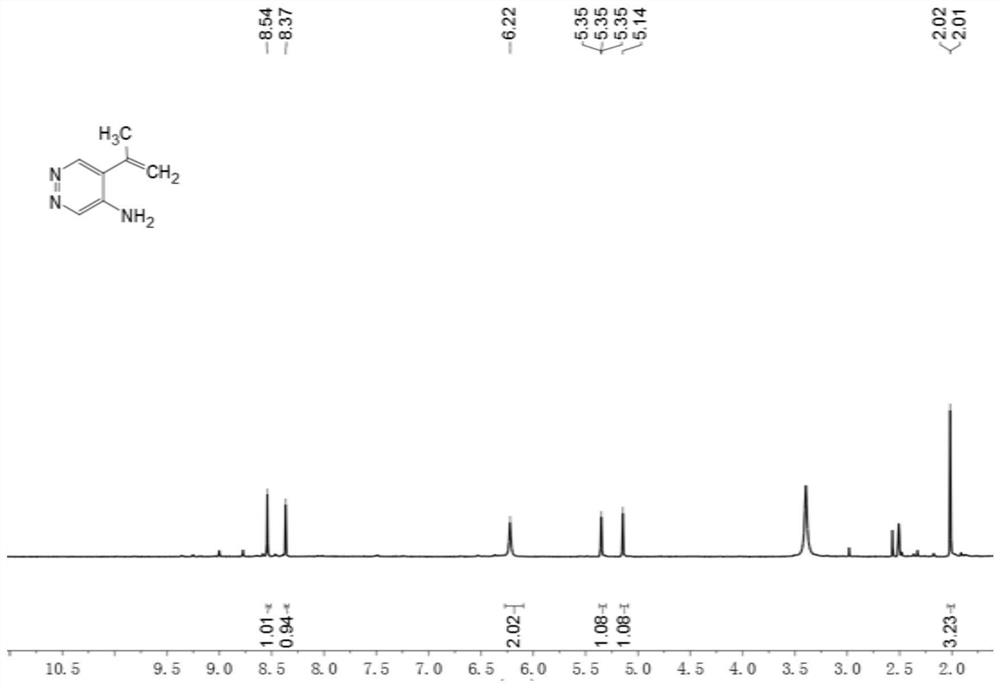
[0056] Example 1 Synthesis of 5-(1-propen-2-yl)pyridine pyridazin-4-amine, whose structural formula is shown in (Ia),
[0057]
[0058] The compound of present embodiment structural formula as shown in (Ia), its preparation method is:
[0059] 1) Dissolve 5-bromopyridazin-4-amine (3) (1.7g, 10.0mmol) in a mixed solution of N,N-dimethylformamide (30mL) and water (5mL) under nitrogen protection , to obtain the mixture A;
[0060] 2) Then add isopropenylboronic acid pinacol ester (2.4g, 14.0mmol), sodium carbonate (2.1g, 20.0mmol) and Pd(dppf)Cl to the mixture A 2 (366mg, 0.50mmol), the mixture was stirred at 100°C for 2 hours; a mixture B was obtained;
[0061] 3) Then the mixed liquid B was diluted with water (60 mL), extracted with ethyl acetate (3×100 mL), the organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and then concentrated under reduced pressure to obtain a crude product;
[0062] 4) The crude product was separated and purified ...
Embodiment 2
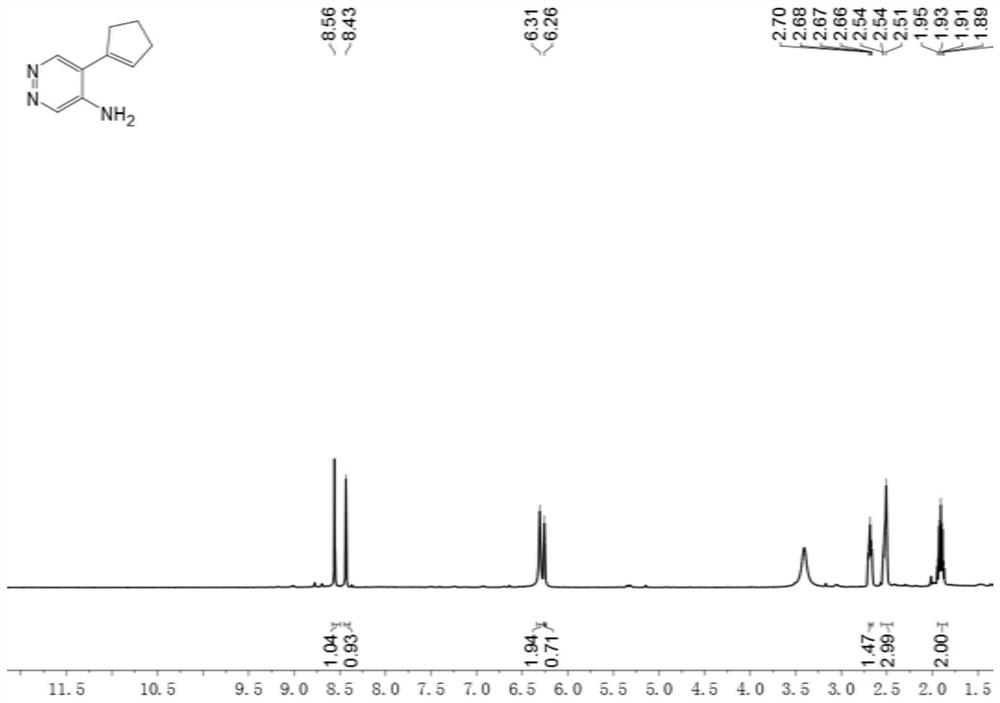
[0065] Example 2 Synthesis of 5-(cyclopent-1-en-1-yl)pyridazin-4-amine, whose structural formula is shown in (Ib),
[0066]
[0067] In the present embodiment, the preparation method of the compound shown in structural formula as (Ib), comprises:
[0068] 1) Dissolve 5-bromopyridazin-4-amine (3) (1.7g, 10.0mmol) in a mixed solution of N,N-dimethylformamide (30mL) and water (5mL) under nitrogen protection , to obtain the mixture A;
[0069] 2) Then add 1-cyclopenteneboronic acid pinacol ester (2.7g, 14.0mmol), sodium carbonate (2.1g, 20.0mmol) and Pd(dppf)Cl in the mixed solution A 2 (366mg, 0.50mmol), the mixture was stirred at 100°C for 2 hours to obtain a mixed solution B;
[0070] 3) Then add water (60mL) to the mixture B to dilute, extract with ethyl acetate (3×100mL), wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, and then concentrate under reduced pressure to obtain a crude product;
[0071] 4) The crude product was separated and pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com