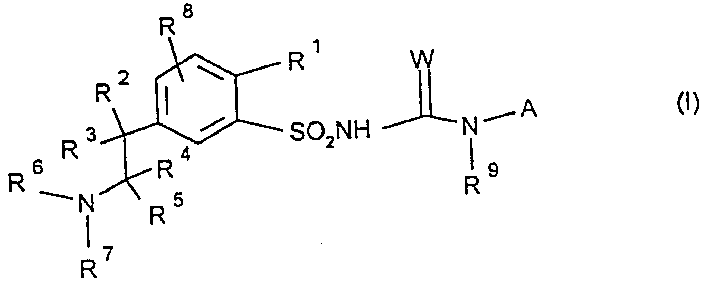
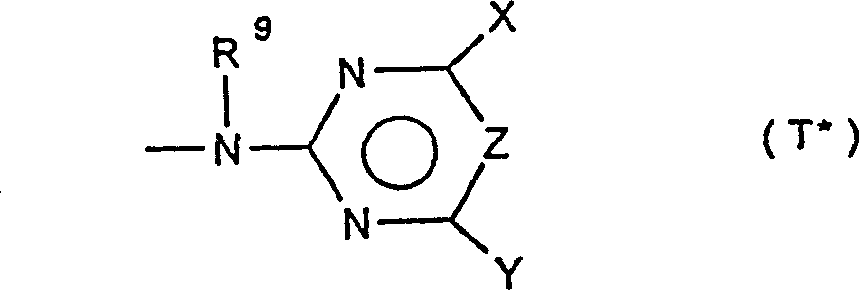
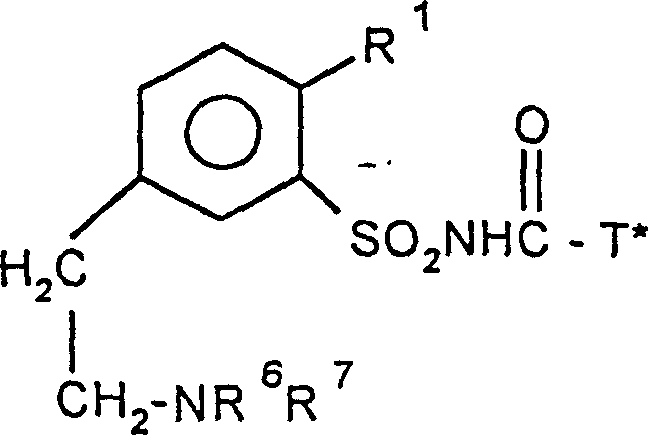
Phenylsulphonyl ureas, processes for their preparation and their use as herbicides and plant-growth regulators
An alkyl and group technology, which is applied in the field of phenylsulfonylurea herbicides and plant growth regulators, can solve problems such as insufficient selectivity and high persistence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A12
[0078] Embodiment A12-(tert-butylaminosulfonyl)-4-cyanomethylbenzoic acid methyl ester
[0079] 100 grams of 61.5% 2-(tert-butylaminosulfonyl)-4-bromomethylbenzoic acid methyl ester (61.5 grams = 0.169 moles), 12.09 grams (0.186 moles) of potassium cyanide and 10.89 grams (33.8 mg mol) of tetrabutylammonium bromide in 750 ml of dichloromethane and 150 ml of water was stirred at room temperature for 6 hours.
[0080] The mixture was diluted with water, the phases were separated and the aqueous phase was extracted twice with dichloromethane for further processing. The combined organic extracts were dried over sodium sulfate. The crude product obtained by concentration is chromatographed on silica gel with an ethyl acetate / petroleum ether mixture (1:2, v / v) as eluent. Concentration of the fractions with RF=0.18 afforded 48.1 g (91.8%) of methyl 2-(tert-butylaminosulfonyl)-4-cyanomethylbenzoate, melting point 86-88°C. Embodiment A24-(2-aminoethyl)-2-tert-butylaminosulfonylbenzo...
Embodiment A2
[0080] The mixture was diluted with water, the phases were separated and the aqueous phase was extracted twice with dichloromethane for further processing. The combined organic extracts were dried over sodium sulfate. The crude product obtained by concentration is chromatographed on silica gel with an ethyl acetate / petroleum ether mixture (1:2, v / v) as eluent. Concentration of the fractions with RF=0.18 afforded 48.1 g (91.8%) of methyl 2-(tert-butylaminosulfonyl)-4-cyanomethylbenzoate, melting point 86-88°C. Embodiment A24-(2-aminoethyl)-2-tert-butylaminosulfonylbenzoic acid methyl ester
[0081] A solution of 2.5 g (8.05 mmol) of methyl 2-(tert-butylaminosulfonyl)-4-cyanomethylbenzoate in 200 ml of methanol and 4 ml of concentrated hydrochloric acid was added to the Hydrogenation was carried out at room temperature under 20 bar hydrogen pressure for 10 hours. The catalyst was filtered off and the filtrate was concentrated. After taking up the residue in ethyl acetate, th...
Embodiment A5
[0092] A solution of 1.4 g (3.8 mmol) of methyl 2-tert-butylaminosulfonyl-4-[2-(methoxycarbonylamino)ethyl]benzoate in 20 ml of trifluoroacetic acid was stirred at room temperature for 8 Hour. The mixture was concentrated well, reevaporated with toluene and the residue was crystallized from ethyl acetate / diisopropyl ether. 0.54 g (45%) of methyl 2-aminosulfonyl-4-[2-(methoxycarbonylamino)ethyl]benzoate of melting point: 146 to 149° C. are obtained. Example A5 2-[(4,6-dimethoxypyrimidin-2-yl)aminocarbonylaminosulfonyl]-4-[2-(methoxycarbonylamino)ethyl]benzoic acid methyl ester
[0093] In 0.54 g (1.7 mmol) of 2-aminosulfonyl-4-[2-(methoxycarbonylamino) ethyl]benzoic acid methyl ester and 0.47 g (1.7 mmol) of N-(4,6-dimethyl To a suspension of oxypyrimidin-2-yl)phenylcarbamate in 15 ml acetonitrile was added dropwise 0.26 ml (1.7 mmol) DBU. after 2 hours. Dilute with water and diethyl ether, acidify to pH 1 to 2 with hydrochloric acid, filter the precipitated product, wash w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com