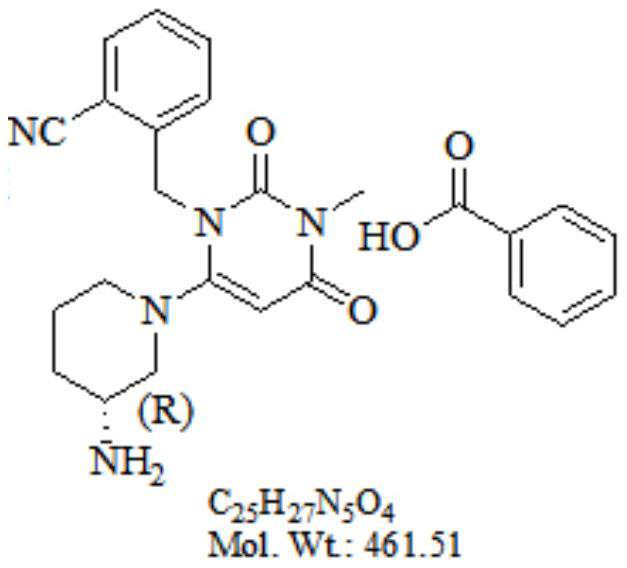
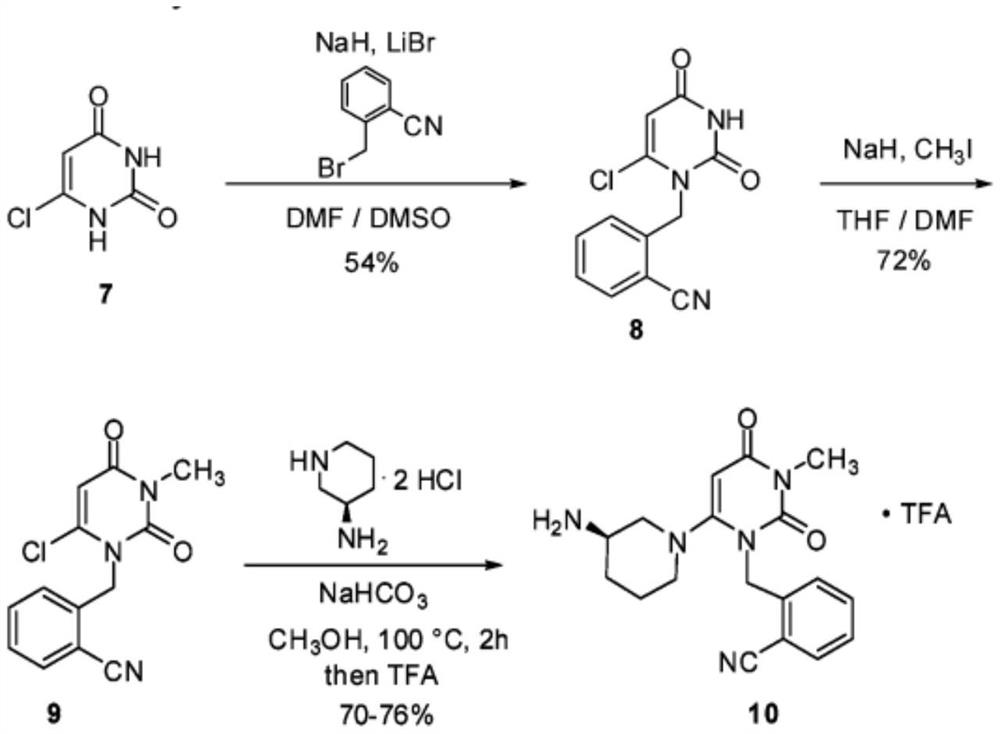
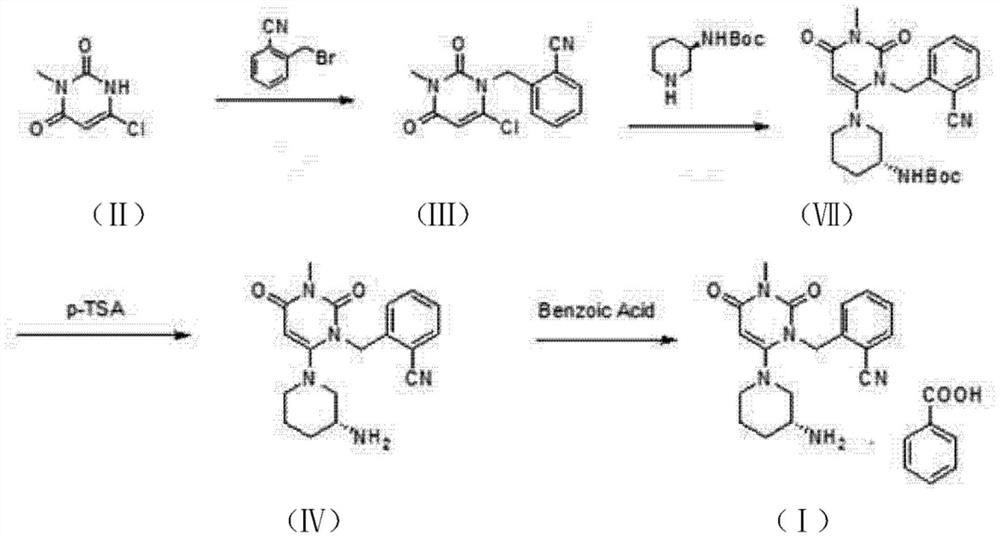
Preparation method of alogliptin benzoate with high yield
A benzoic acid, high-yield technology, applied in the field of pharmaceutical preparation, can solve the problems of increasing other impurity risks, increasing reaction steps, increasing costs, etc., and achieves the effects of simplified quality control inspection means, less impurities, and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation Intermediate 2- (6-chloro-3-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl - methyl) -4-fluorobenzene Methitrile
[0031]
[0032] N, N-dimethylformamide 20g, 6-chloro-3-methyl uracil 5.1 g, 2-cyano-3-methyl uracil 5.1 g, 2-cyano-3-methyl uracil, N-diisopropylamine 4g, heating To 45-75 ° C, the reaction time is 2 to 8 h. Cooling, adding 55 kg, stirring temperature 10 to 20 ° C, filtration to obtain solid, dried to give 8.5 g of product, yield 95%.
Embodiment 2
[0033] Example 2: Preparation of agellatine hydrochloride
[0034]
[0035] 50 g of acetonitrile 50g, intermediate I 8.3 g, (R) -3-amino piperidiniol, 18g, 18 g of sodium hydrogencaridin, temperature rise to acetonitrile, refluxed to acetonitrile, refluxed to 20 ~ 8 hours, cool down to 20- 30 ° C, filtration, filtrate was added to the reaction bottle to cool down to 0 to 10 ° C, and concentrated hydrochloric acid, filtered, dried to give a white solid 10.2 g, yield 90%.
Embodiment 3
[0036] Example 3: Preparation of benzoic acid Agelipine
[0037]
[0038] Agelipatine hydrochloride 10g and purified water 80 g were added to the reaction flask, and pH to 7.0 to 9.5 were adjusted with sodium bicarbonate. The organic phase was separated, and the aqueous phase was added to methylenechloromethane to extract Agelipine. The organic phase was evaporated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com