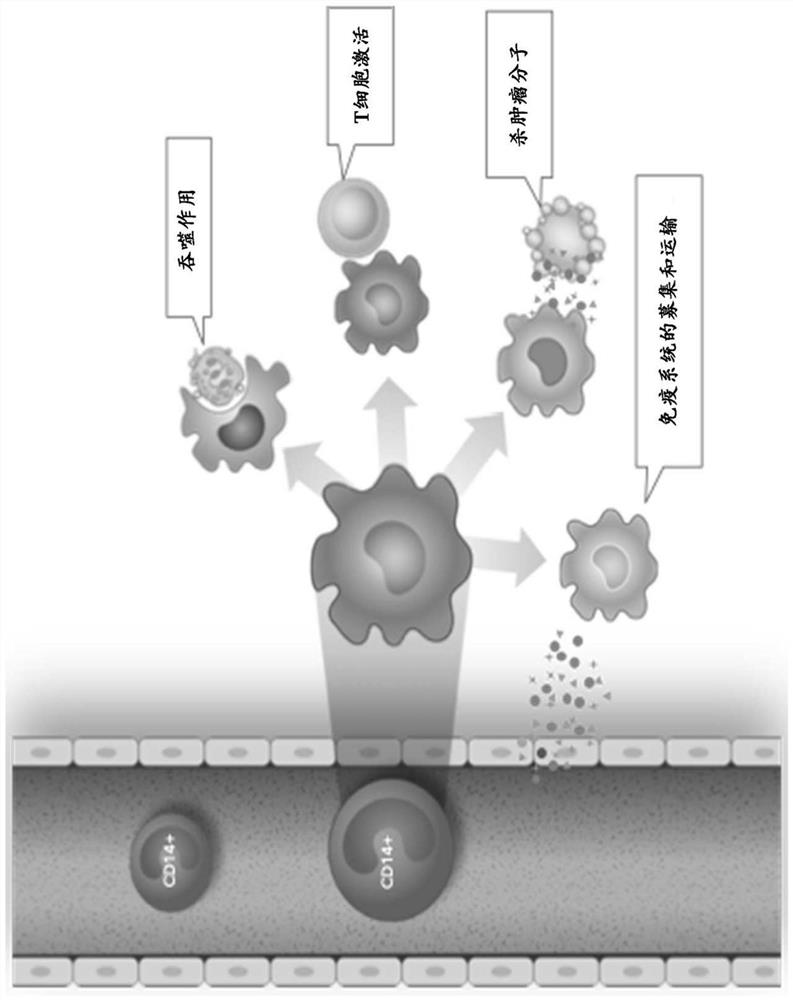
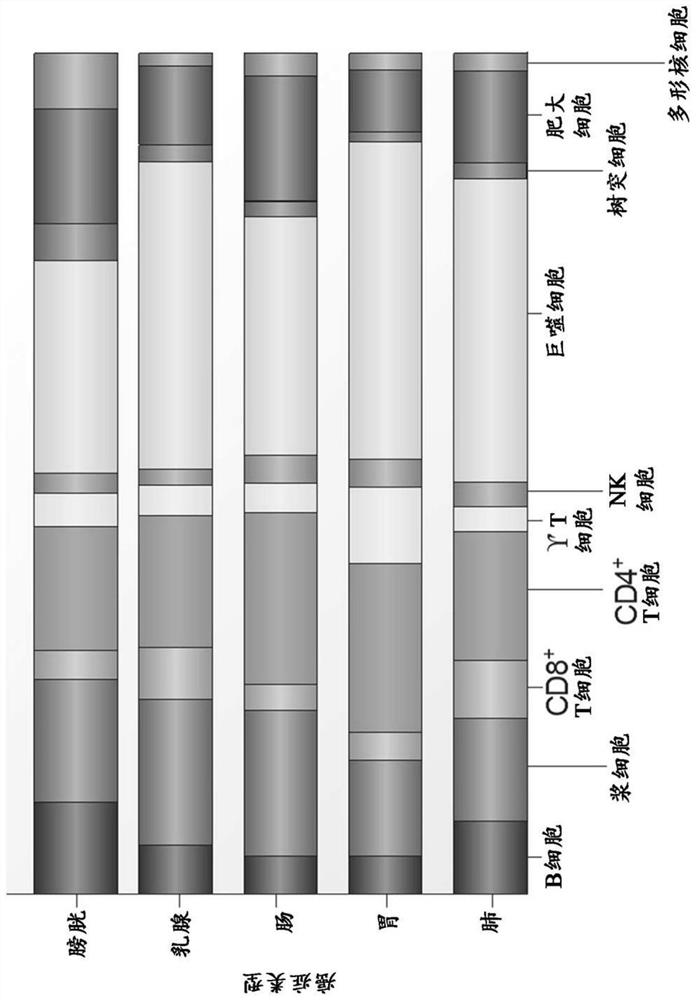
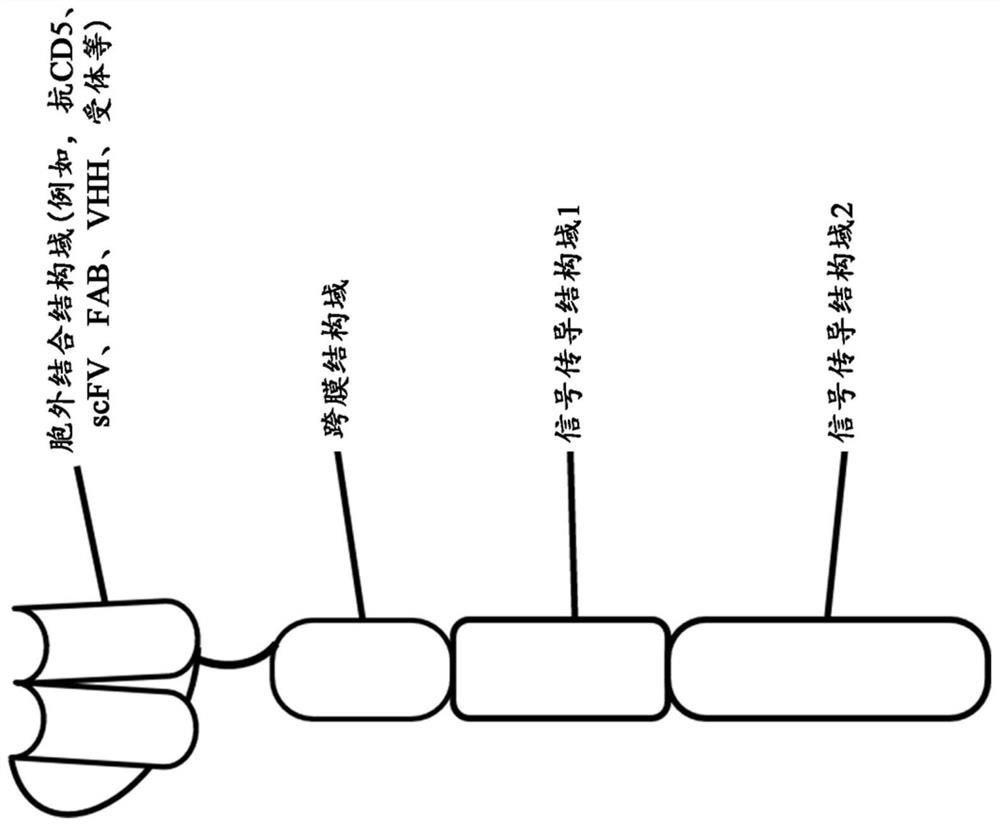
Engineered chimeric fusion protein compositions and methods of use thereof
A technology of fusion protein and composition, applied in obstacles, one purpose is to use myeloid cells to promote, destroy the integrity of nucleic acid and make gene transfer into these cells an inefficient field, able to solve the obstacles of viral transduction, not applicable issues of gene transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0449] The preparation method of CAR-P comprises the following steps: (1) screening PSR subunit framework; (2) screening antigen binding specificity; (3) CAR-P recombinant nucleic acid construct; (4) engineered cells and verification.
[0450] Screening for PSR subunit frameworks: As described above, receptors were designed to include at least one phagocytic receptor domain capable of enhancing signaling for phagocytosis. Essentially, a large number of plasma membrane proteins can be screened for novel phagocytosis function or enhancing domains. Methods of screening for phagocyte receptor subunits are known to those skilled in the art. Additional information can be found in the Examples section. Generally speaking, functional genomics and reverse engineering are usually used to obtain gene sequences encoding functionally related protein polypeptides or parts thereof. In some embodiments, primers and probes are constructed for the identification and / or isolation of proteins, ...
Embodiment approach
[0751] 1. A composition comprising a recombinant nucleic acid encoding a phagocyte or a tethered receptor (PR) fusion protein (PFP), said phagocytic cell or a tethered receptor (PR) fusion protein (PFP) comprising: (a) A PR subunit comprising: (i) a transmembrane domain, and (ii) an intracellular domain comprising an intracellular signaling domain; (b) comprising an antigen binding domain specific for a target cell antigen wherein the transmembrane domain and the extracellular domain are operably linked; and wherein when the PFP binds to an antigen of the target cell, it is associated with a cell that does not express the PFP Compared, the killing or phagocytic activity of cells expressing said PFP is increased by at least greater than 20%.
[0752] 2. The composition of embodiment 1, wherein the intracellular signaling domain is derived from a phagocyte or a tethered receptor, or wherein the intracellular signaling domain comprises a phagocytosis activation domain.
[0753] ...
Embodiment 1
[0980] Example 1. Generation of Novel Chimeric Receptor Fusion Protein (CFP) Constructs
[0981] In this section, exemplary designs for identifying useful CFP ECD, TM, ICD and antigen-binding domains for generating novel CFPs are described. Briefly, a large number of potential candidate proteins were screened for enhanced phagocytic properties and their respective phagocytosis-related intracellular signaling. The useful domains are then used to generate novel CFPs. Screening can be divided into two parts: A. Screening of PR domain; B. Screening of extracellular antigen-binding domain.
[0982] Screening for PR domains:
[0983] 5,800 plasma membrane proteins were screened for their phagocytic potential. J774 macrophages were transiently transfected with a library of 5800 plasma proteins. High-throughput multiplex assays (ranging from 6-well plate assay set-up to 384-well plate assays with robotic handling) were established to assess various potential functions of the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com