Application of iron chelating agent in preparation of medicine for treating or preventing polyomavirus infection
A polyoma virus and iron chelating agent technology, applied in the preparation of medicines for treating or preventing polyoma virus infection or inhibiting polyoma virus, the field of iron chelating agents can solve the problem that no iron chelating agent has been found to treat or prevent polyoma virus-related diseases And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Embodiment 1: the inhibitory effect research of iron chelating agent to BK virus
[0190] The purpose of this example is to study the inhibitory effect of iron chelators on BK virus (BKV) in cells.
[0191] 1.1 Dissolve deferasirox (DFS), deferoxamine (DFA) and deferiprone (DFP) in DMSO or PBS, respectively, and prepare 250mM, 100mM and 100mM concentration storage solutions for subsequent use .
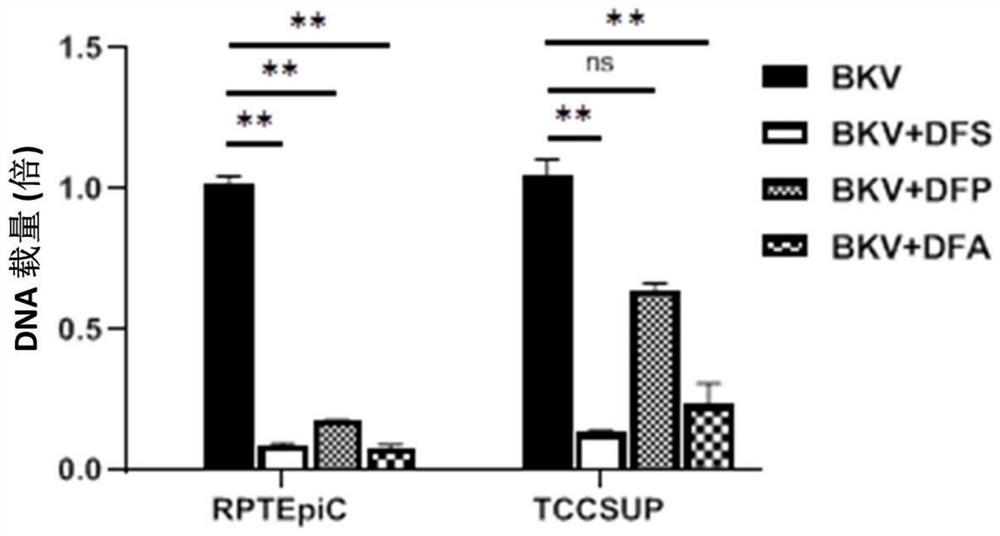
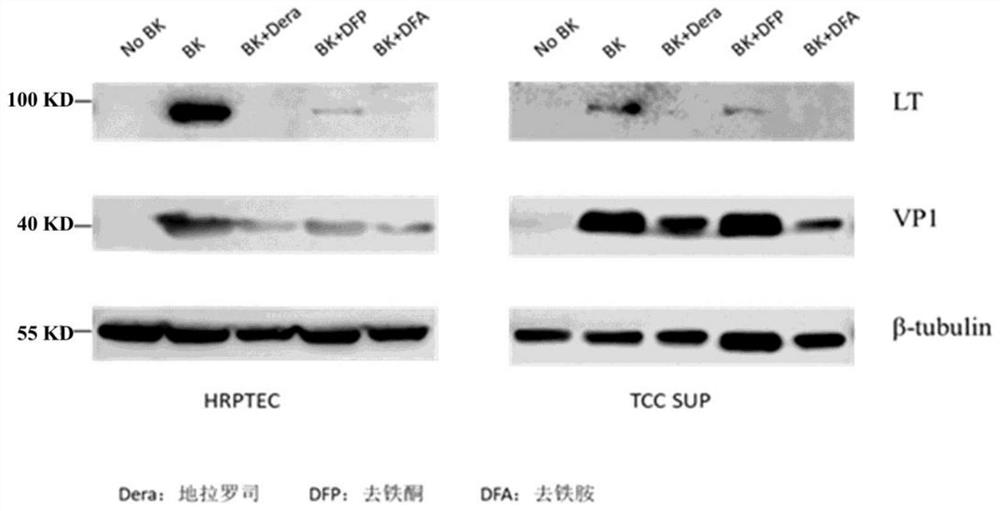
[0192] 1.2 Use 0.25% Trypsin-0.1% EDTA to digest HRPTEPIC and TCCSUP cells in the logarithmic phase at 3×10 5 The number of cells / well was seeded into 6-well plate, 2ml per well. The above two types of cells were infected within 24 hours of the cell seeding plate, and the cell supernatant in the 6-well plate was aspirated as much as possible, and replaced with the corresponding medium containing 0.5 MOI BKV. After 2 hours of infection, the supernatant was discarded and replaced with a drug-free medium and a medium containing 100 μM deferasirox, 100 μM deferoxamine, and 100 μ...
Embodiment 2
[0198] Embodiment 2: the research of iron chelating agent anti-BK virus effect and iron ion level
[0199] The purpose of this example is to study the inhibitory effect of iron chelators on BK virus in the presence of iron ions.
[0200] 2.1 Dissolve deferasirox, deferoxamine, and deferiprone in DMSO or PBS, respectively, and prepare stock solutions at concentrations of 250 mM, 100 mM, and 100 mM, respectively, for subsequent use.
[0201] 2.2 Use 0.25% Trypsin-0.1% EDTA to digest HRPTEPIC and TCCSUP cells in the logarithmic phase at 3×10 5 The number of cells / well was seeded into 6-well plate, 2ml per well. The above two types of cells were infected within 24 hours of the cell seeding plate, and the cell supernatant in the 6-well plate was aspirated as much as possible, and replaced with the corresponding medium containing 0.5 MOI BKV. After 2 hours of infection, the supernatant was discarded, and replaced with a drug-free medium and a medium containing 10 μM deferasirox, 2...
Embodiment 3
[0207] Embodiment 3: the research of iron chelator treatment or prevention BK virus-associated disease
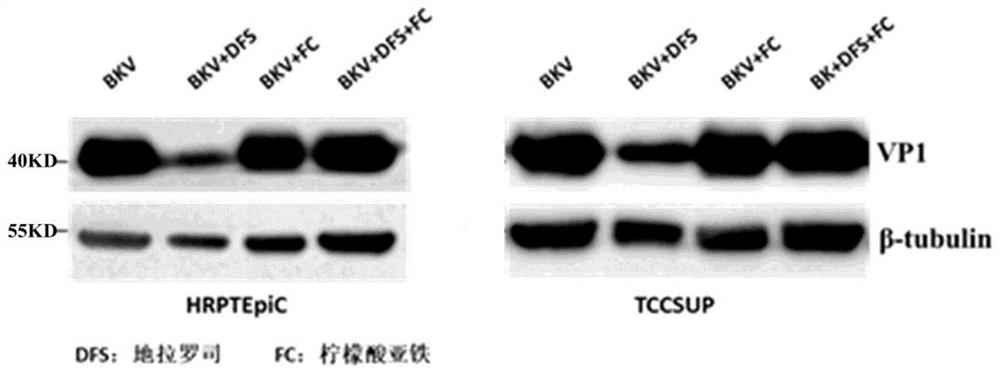
[0208] The purpose of this example is to study the inhibitory effect of iron chelators on BK virus in BKV recipient cells by administering iron chelators before and after BK virus infection of cells.
[0209] 3.1 Dissolve deferasirox, deferoxamine, and deferiprone in DMSO or PBS, respectively, and prepare stock solutions at concentrations of 250 mM, 100 mM, and 100 mM, respectively, for subsequent use.
[0210] 3.2 Use 0.25% Trypsin-0.1% EDTA to digest TCCSUP cells grown in logarithmic phase at 3×10 5 The number of cells per well was seeded into 6-well plates and divided into 4 groups. The above-mentioned cells were infected within 24 hours of the cell seed plate, and the cell supernatant in the 6-well plate was blotted as much as possible. The above four groups were replaced with BK virus, BK virus and 25 μM deferasirox, BK virus and 25 μM deferasirox, respectively. 25 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com