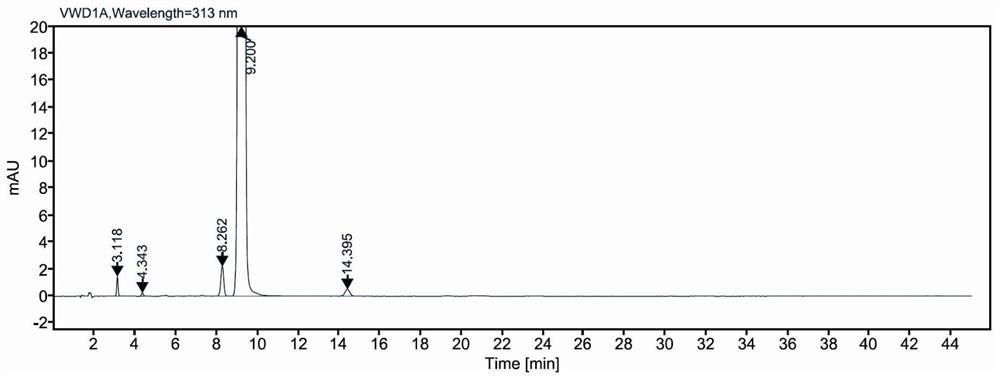
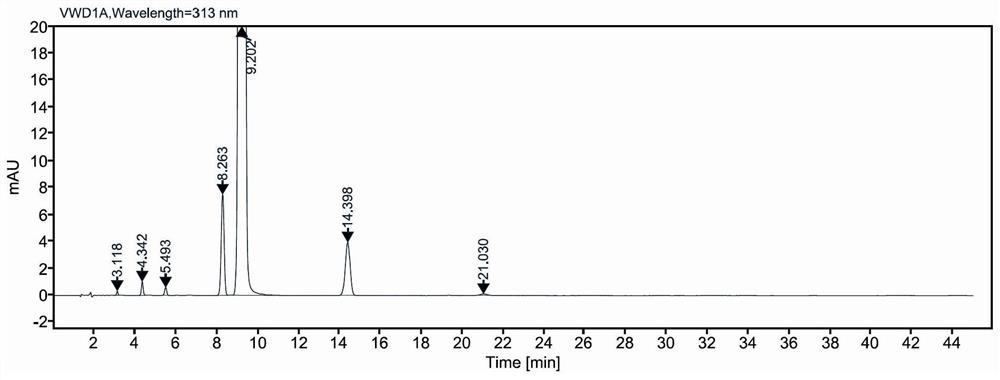
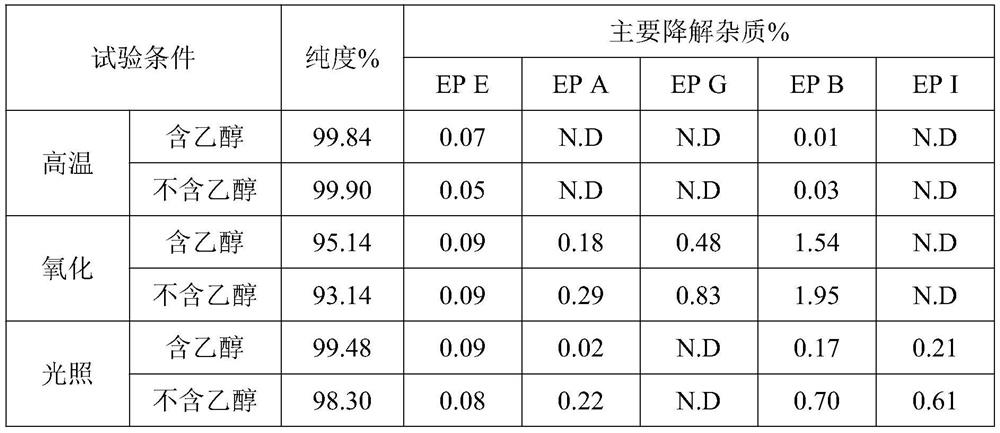
Low-irritation ketorolac tromethamine injection with stable chemical property
A technology of irritating ketorolac tromethamine and ketorolac tromethamine, applied in the direction of organic active ingredients, non-central analgesics, medical preparations of non-active ingredients, etc., can solve the irritating effect of potassium salts , did not solve the problems of irritation and complex ingredients, and achieved the effects of stable quality, reduced risk of phlebitis, and increased drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] (1) Prescription composition
[0074] Ketorolac tromethamine 30g Sodium chloride 4.35g sodium hydroxide Appropriate amount, adjusted to pH about 7.4 Water for Injection to 1L batch 1000 pieces inner packaging container 1mL amber glass ampoule
[0075] (2) Preparation method:
[0076] 1. Add sodium chloride to 90% prescription water for injection cooled to room temperature, and stir until completely dissolved;
[0077] 2. Add ketorolac tromethamine and stir until completely dissolved;
[0078] 3. Add 2M sodium hydroxide aqueous solution to adjust the pH of the liquid to about 7.39;
[0079] 4. Use the remaining amount of water for injection to adjust the concentration of ketorolac tromethamine to 30mg / mL;
[0080] 5. The liquid medicine is filtered through a 0.2μm filter element;
[0081] 6. Filling and sealing under nitrogen protection;
[0082] 7. Moist heat sterilization, 121°C for 15 minutes;
[0083] 8. Visual in...
Embodiment 2
[0086] (1) Prescription composition
[0087] Ketorolac tromethamine 30g Sodium chloride 4.35g sodium hydroxide Appropriate amount, adjusted to pH about 7.4 citric acid 5g Water for Injection to 1L batch 1000 pieces inner packaging container 1mL amber glass ampoule
[0088] (2) Preparation method:
[0089] 1. Add sodium chloride and citric acid to 90% prescription water for injection cooled to room temperature, and stir until completely dissolved;
[0090] 2. Add ketorolac tromethamine and stir until completely dissolved;
[0091] 3. Add 2M sodium hydroxide aqueous solution to adjust the pH of the liquid to about 7.40;
[0092] 4. Use the remaining amount of water for injection to adjust the concentration of ketorolac tromethamine to 30mg / mL;
[0093] 5. The liquid medicine is filtered through a 0.2μm filter element;
[0094] 6. Filling and sealing under nitrogen protection;
[0095] 7. Moist heat sterilization, 121°C f...
Embodiment 3
[0099] (1) Prescription composition
[0100] Ketorolac tromethamine 15g Sodium chloride 6.68g sodium hydroxide Appropriate amount, adjusted to pH about 7.4 citric acid 5g Water for Injection to 1L batch 1000 pieces inner packaging container 2mL amber glass tube injection bottle, bromobutyl rubber stopper
[0101] (2) Preparation method:
[0102] 1. Add sodium chloride and citric acid to 90% prescription water for injection cooled to room temperature, and stir until completely dissolved;
[0103] 2. Add ketorolac tromethamine and stir until completely dissolved;
[0104] 3. Add 2M sodium hydroxide aqueous solution to adjust the pH of the liquid to about 7.40;
[0105] 4. Use the remaining amount of water for injection to adjust the concentration of ketorolac tromethamine to 15 mg / mL;
[0106] 5. The liquid medicine is filtered through a 0.2μm filter element;
[0107] 6. Filling, stoppering and capping under nitrogen prot...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap