Preparation method of 2-methyl-5-(1-methylpyrrolidine-2-yl) pyridine
A technology of methylpyrrolidine and alkenylpyrrolidone, which is applied in the field of preparation of 2-methyl-5-pyridine, can solve the problems of high cost, low output, potential danger to human health, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
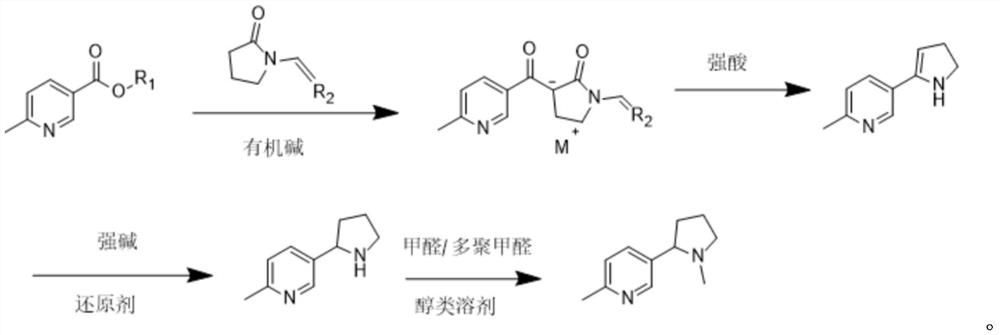
[0031] (1) Take 400g of methyl 6-methylnicotinate, 300g of vinylpyrrolidone, and 255g of sodium tert-butoxide. Under the protection of nitrogen, add 2L of toluene and mix well, then heat to 110°C and reflux. The product is precipitated in solid form and passed through LC -MS monitors the completion of the reaction. After 8 hours of reaction, the toluene solvent is filtered, washed three times with 300g of petroleum ether, and dried at 30°C for 2 hours to obtain 1-methyl-3-(6-methyl-nicotinoyl)-2- Vinylpyrrolidone salt 583g.
[0032] (2) 583g of 1-methyl-3-(6-methyl-nicotinoyl)-2-vinylpyrrolidone salt was obtained in step (1), added 1.25kg of 36% concentrated hydrochloric acid, heated to 88°C, and passed LC-MS Monitor the completion of the reaction and react for 44h.
[0033] (3) Cool down to -20°C, add 40% potassium hydroxide aqueous solution to adjust the pH to 11, add 570g of sodium dithionite solid, stir and react at 75°C, monitor the completion of the reaction by LC-MS, a...
Embodiment 2
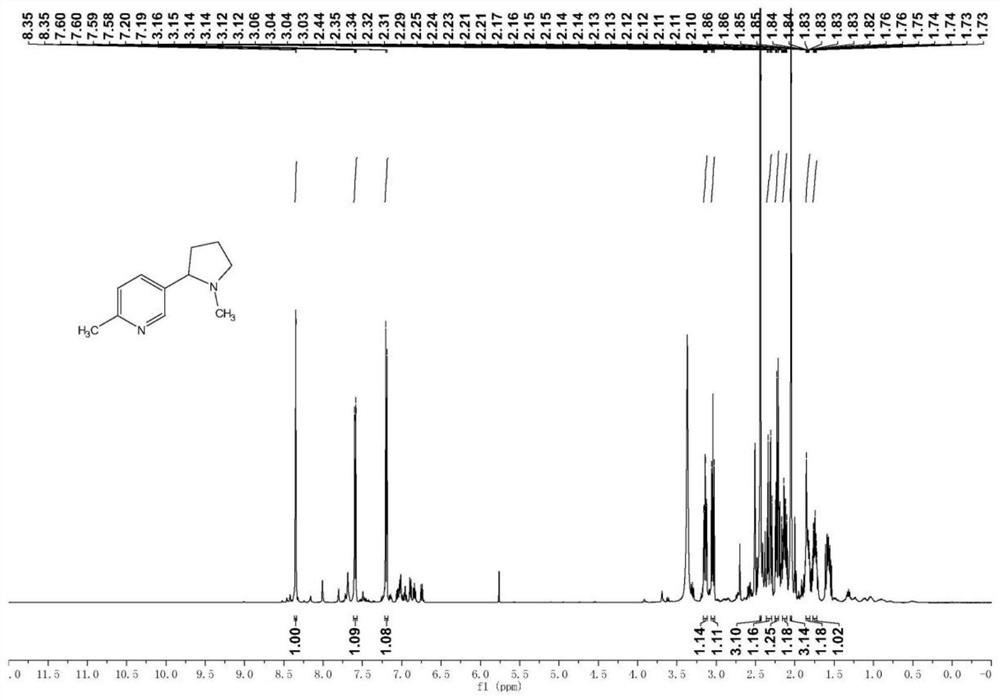
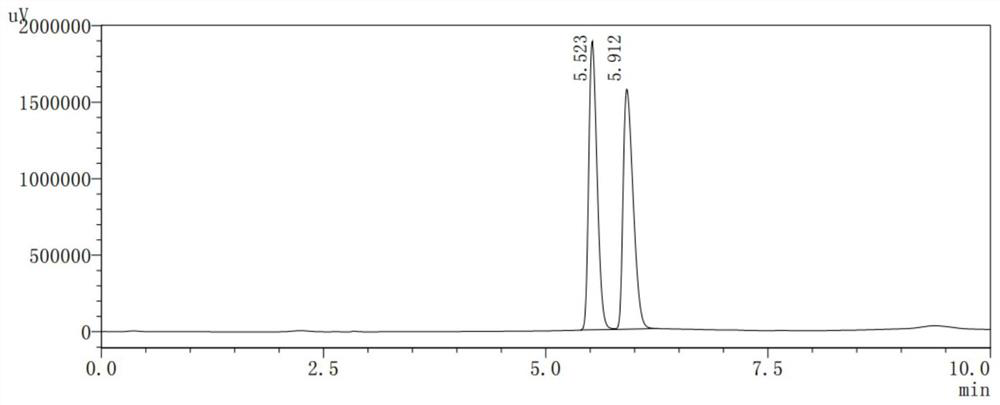
[0041] Other conditions and operations were the same as in Example 1, except that the reducing agent in step 3 was 343g lithium triethylborohydride, and step 3 obtained 263g of 2-methyl-5-(pyrrolidin-2-yl)pyridine. Finally, 235 g of pure 2-methyl-5-(1-methylpyrrolidin-2-yl)pyridine was obtained, with a GC purity of 99.2%. Based on 6-methylnicotinic acid methyl ester, the total yield was 50.00%.
Embodiment 3
[0043] Other conditions and operation are identical with embodiment 1, and difference is that reducing agent is 443g sodium dithionite and 77g lithium triethylborohydride in step 3, and step 3 obtains 2-methyl-5-(pyrrolidin-2-yl)pyridine 307g. Finally, 276 g of pure 2-methyl-5-(1-methylpyrrolidin-2-yl)pyridine was obtained, with a GC purity of 99.3%. Based on 6-methylnicotinic acid methyl ester, the total yield was 58.78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com