Method for comprehensively evaluating adenovirus pAd-M3C titer by combining TCID50, MOI, GTU and PFU
A comprehensive evaluation and virus titer technology, applied in the field of molecular biology and cellular immunity, can solve the problems of no clear standards for mutual conversion, achieve the effect of reducing experimental costs, improving reliability and stability, and reducing repeated experiments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
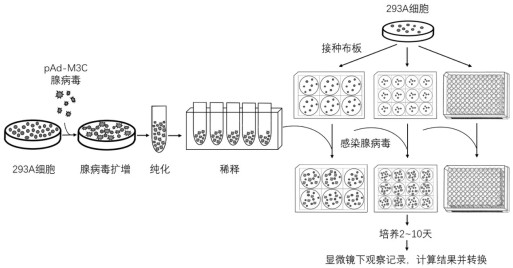
[0050] Example 1 TCID50, MOI, GTU combined with PFU method for comprehensive evaluation of adenovirus pAd-M3C titer
[0051] (1) The adenovirus pAd-M3C (purchased from Addgene) was massively amplified using the tool cell 293A.
[0052] (2) The adenovirus harvested after amplification was purified using a concentration kit (Vivapure®AdenoPACK™ SARTORIUS (Sartorius, Cat. No. VS-AVPQ 022)).
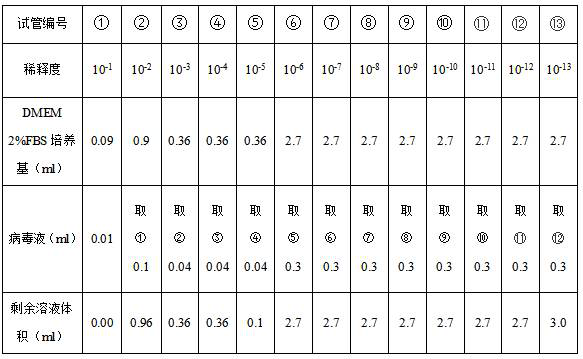
[0053] (3) Carry out titer detection to the purified virus solution:
[0054] (1) 293A cells were seeded in 6-, 12-, and 96-well plates according to the following counting requirements, at 37°C, 5% CO 2 The cells were cultured overnight in an incubator.
[0055] 1) Use DMEM 10wt% FBS medium to prepare at least 3×10 7 The cells were prepared with DMEM 2wt% FBS medium for 10 5 cells / ml at least 20 ml.
[0056] 2) Adding samples: Add 293A cells to each well of 6, 12, and 96-well plates according to the following requirements, and repeat for 1 plate:
[0057] 6-well plate: 1×10 6 cells / 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com