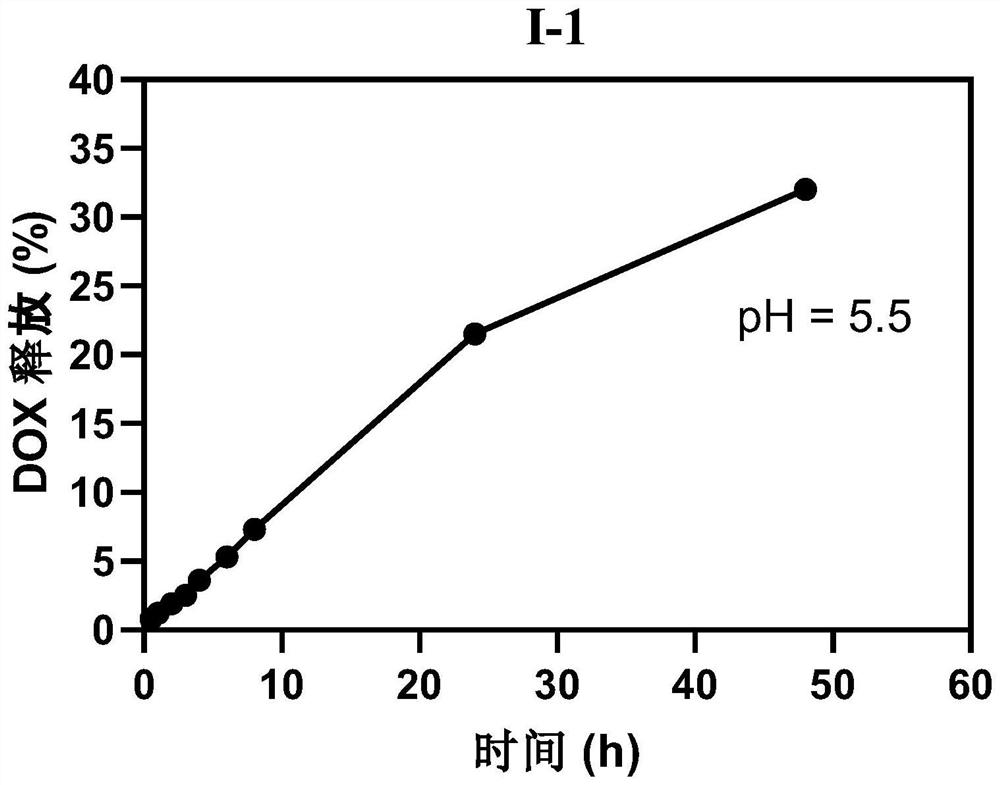
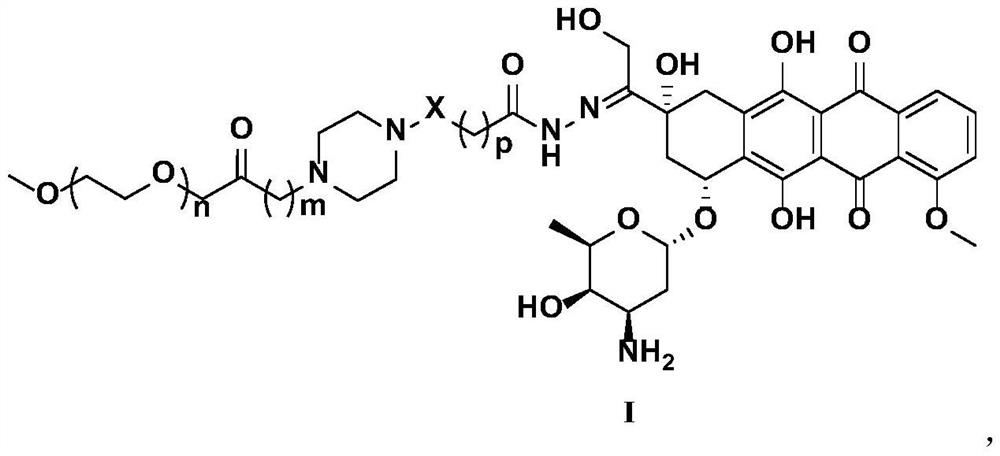
Polyethylene glycol modified anti-tumor prodrug as well as preparation method and application thereof
A polyethylene glycol and prodrug technology, applied in the field of doxorubicin prodrug and its preparation, can solve the problem of entering tumor tissue and the like, and achieve the effects of simple operation, environmental friendliness and excellent release behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: the preparation of intermediate 2a
[0086]
[0087] Put tert-butyl carbazate (2g, 1eq), succinic anhydride (1eq) and water (15mL) in a 100mL round bottom flask and stir at room temperature for 5h. Freeze-dried to obtain compound 2a, a total of 3.5 g, with a yield of 98%.
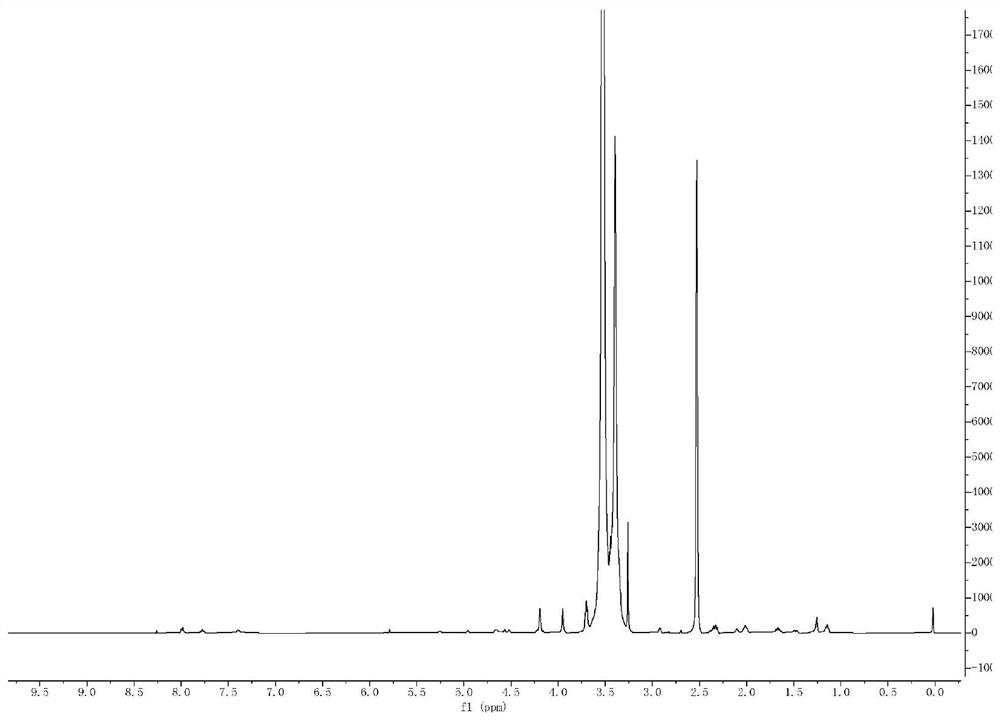
[0088] 1 H NMR (400MHz, CDCl 3 )δ8.72(s,1H),7.35(s,1H),2.69(t,J=6.7Hz,2H),2.54(t,J=6.7Hz,2H),1.45(s,9H).
Embodiment 2
[0089] Embodiment 2: the preparation of intermediate 2b
[0090]
[0091] Put tert-butyl carbazate (2g, 1eq), glutaric anhydride (1eq) and water (15mL) in a 100mL round bottom flask and stir at room temperature for 5h. Compound 2b was freeze-dried to obtain 3.7 g in total, with a yield of 98%.
[0092] 1 H NMR (400MHz, CDCl 3 )δ8.88(s, 1H), 7.17(d, J=3.5Hz, 1H), 2.44(t, J=6.6Hz, 2H), 2.35(t, J=7.3Hz, 2H), 1.98(p, J=6.8Hz,2H),1.46(s,9H).
Embodiment 3
[0093] Embodiment 3: the preparation of intermediate 2c
[0094]
[0095] Put tert-butyl carbazate (2g, 1eq), adipic anhydride (1eq) and water (15mL) in a 100mL round bottom flask and stir at room temperature for 5h. Compound 2c was freeze-dried to obtain 3.7 g in total, with a yield of 95%.
[0096] 1 H NMR (400MHz, CDCl 3 )δ8.76(s,1H),7.19(d,J=3.4Hz,1H),2.49(t,J=6.7Hz,2H),2.37(t,J=6.7Hz,2H),1.88–1.99( m,2H),1.73–1.62(m,2H),1.46(s,9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com