A kind of preparation method of tacrolimus solid dispersion tablet
A technology of tacrolimus and solid dispersion, applied in the field of medicine, to achieve the effect of good application prospect, low production cost and good release uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Batch of 3000 tablets, tablet weight: 253.5mg / tablet.
[0050] Prescription composition:
[0051]
[0052]
[0053] The preparation method is as follows:
[0054] Carbomer 943 and polysorbate were dissolved in purified water, Tween 80 and tacrolimus were dissolved in ethanol, the ethanol solution and aqueous solution were mixed, the homogenizer was dispersed at 2000rpm for 1min, and then stirred at 1000rpm for 5min. Put the suspension into the hopper of the media mill, the medium is zirconium silicate, the particle size is about 1-1.2mm, and the flow rate is about 100ml / min. Grinding time is 30min. Put the lactose and HPMC into the fluidized bed pot after being fully mixed, and spray the ground nanostructured suspension on the second. The air inlet temperature of the fluidized bed is 65°C, the material temperature is controlled at about 55°C, the exhaust frequency is 40%, the atomization pressure is 0.15mpa, and the liquid supply rate is 0.6g / min. Compressed w...
Embodiment 2
[0059] Batch of 3000 tablets, tablet weight: 253.5mg / tablet.
[0060] Prescription composition:
[0061]
[0062]
[0063] The preparation method is as follows:
[0064] Preparation method is with embodiment 1.
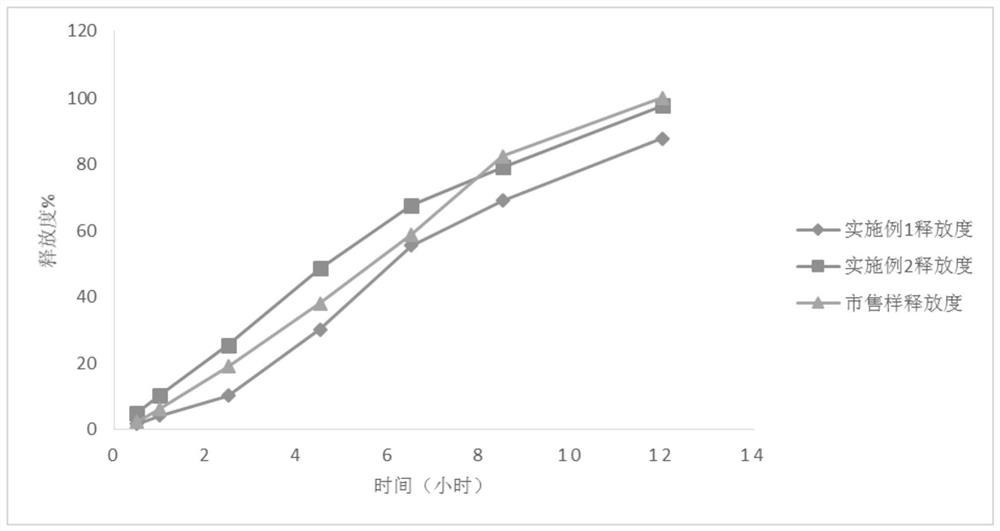
[0065] Embodiment 1, the degree of release of 2
[0066] time Embodiment 1 release degree Embodiment 2 release degree commercial sample release 0.5h 1.72 5.07 2.42 1h 4.36 10.49 6.35 2.5h 10.22 25.61 19.07 4.5h 30.28 48.63 38.08 6.5h 55.63 67.76 58.87 8.5h 69.20 79.23 82.53 12h 87.91 97.77 100.28
[0067] See attached figure 1 .
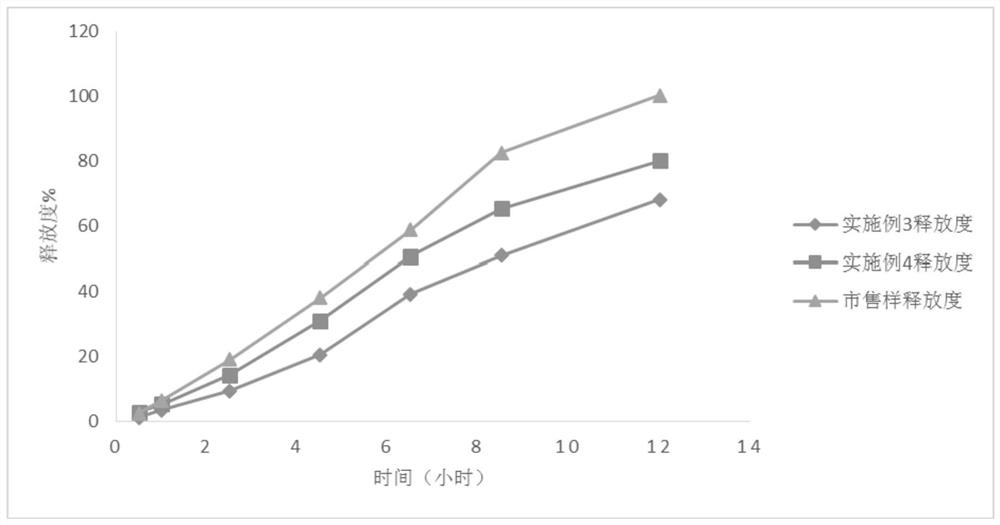
Embodiment 3
[0069] Batch of 4000 tablets, tablet weight: 203mg / tablet.
[0070] Prescription composition:
[0071]
[0072]
[0073] The preparation method is as follows:
[0074] Dissolve CMC-Na and PEG6000 in purified water, dissolve poloxamer 188 and tacrolimus in ethanol, mix the ethanol solution and aqueous solution, disperse with a homogenizer at 2000rpm for 1min, and then stir at 1000rpm for 5min. Put the suspension into the hopper of the media mill, the medium is zirconium silicate, the particle size is about 1-1.2mm, and the flow rate is about 80ml / min. Grinding time is 40min. After fully mixing the microcrystalline cellulose and HPMC, put them into a fluidized bed pot, and spray the ground nanostructured suspension on the second carrier mixture. The air inlet temperature of the fluidized bed is 60°C, the material temperature is controlled at about 50°C, the exhaust frequency is 45%, the atomization pressure is 0.12mpa, and the liquid supply rate is 0.8g / min. Compressed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com