Targeting lysosome super-resolution self-flashing dye as well as synthesis method and biological application thereof
A synthetic method and lysosome technology, applied in the field of super-resolution fluorescent dyes, can solve problems such as capture difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of intermediate Rho530
[0047]
[0048]Put 3-N,N-bis(allyl)aminophenol (100mg, 0.53mmol) and phthalic anhydride (38mg, 0.26mmol) in 5mL of 1,2-dichlorobenzene, and then gradually heated up to 190°C , and reacted at this temperature for 10h. After the reaction solution was cooled to room temperature, the sample was wet-loaded and separated by silica gel column chromatography (developing solvent: dichloromethane: methanol volume ratio 20:1) to obtain 45 mg of a purple solid with a yield of 32%.
[0049] Its high-resolution mass spectrometry data are as follows:
[0050] HRMS(ESI)m / z[M] + : Calculated value: 491.2335, Experimental value: 491.2339.
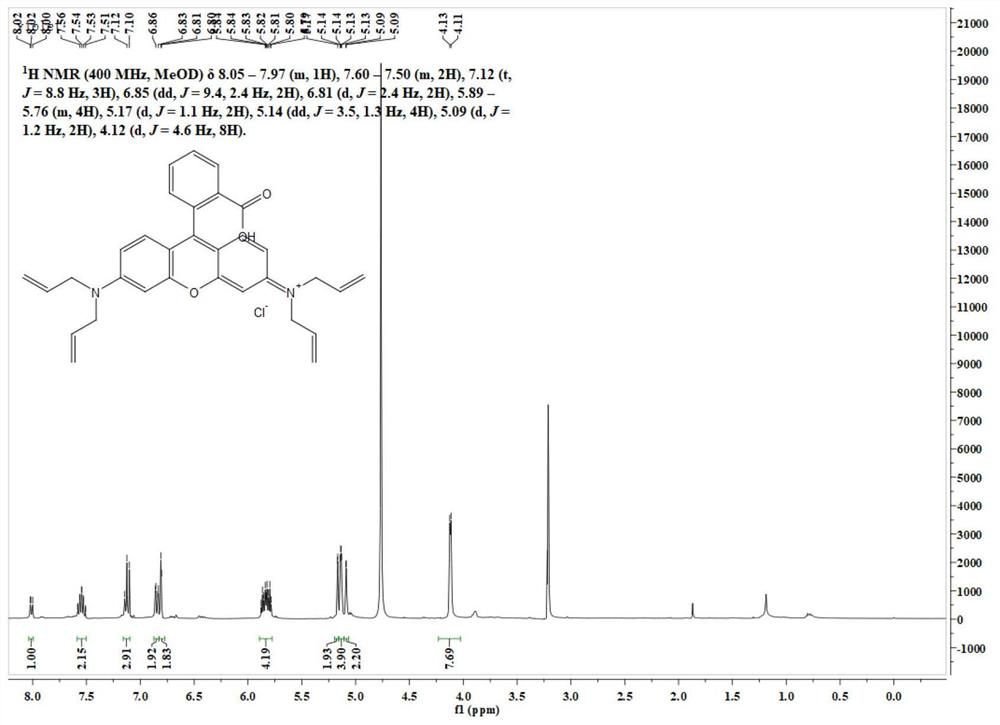
[0051] Its NMR spectrum is as follows figure 1 As shown, the specific data are as follows:
[0052] 1 H NMR (400MHz, MeOD) δ8.05–7.97(m,1H),7.60–7.50(m,2H),7.12(t,J=8.8Hz,3H),6.85(dd,J=9.4,2.4Hz, 2H), 6.81(d, J=2.4Hz, 2H), 5.89–5.76(m, 4H), 5.17(d, J=1.1Hz, 2H), 5.14(dd, J=3.5, 1.3Hz, 4H), 5.09(d, J=1.2Hz,...
Embodiment 2
[0081] Synthesis of intermediate Rho530
[0082]
[0083] Put 3-N,N-bis(allyl)aminophenol (300mg, 1.59mmol) and phthalic anhydride (114mg, 0.77mmol) in 30mL of 1,2-dichlorobenzene, then gradually heat up to 190°C , and react at this temperature for 6h. After the reaction solution was cooled to room temperature, the sample was wet-loaded and separated by silica gel column chromatography (developing solvent: dichloromethane:methanol volume ratio 20:1) to obtain 194 mg of a purple solid with a yield of 46%.
[0084] After testing, its structure is shown in the above formula.
[0085] Synthesis of Dye LysoH-530
[0086]
[0087] Rho530 (200mg, 0.38mmol) was dissolved in 20mL of 1,2-dichloroethane, then 2mL of phosphorus oxychloride was added to the reaction solution, and reacted at 80°C for 2h. It was then removed under reduced pressure to obtain a purple crude product. The crude product was dissolved in 20 mL of acetonitrile, and 0.5 mL of triethylamine and 2-aminopyrid...
Embodiment 3
[0104] Synthesis of intermediate Rho530
[0105]
[0106] Put 3-N,N-bis(allyl)aminophenol (200mg, 1.06mmol) and phthalic anhydride (77mg, 0.56mmol) in 15mL of 1,2-dichlorobenzene, then gradually heat up to 190°C , and reacted at this temperature for 8h. After the reaction solution was cooled to room temperature, the sample was wet-loaded and separated by silica gel column chromatography (developing solvent: dichloromethane:methanol volume ratio 20:1) to obtain 118 mg of a purple solid with a yield of 42%.
[0107] After testing, its structure is shown in the above formula.
[0108] Synthesis of Dye LysoN-530
[0109]
[0110] Rho530 (100mg, 0.19mmol) was dissolved in 15mL of 1,2-dichloroethane, then 0.5mL of phosphorus oxychloride was added to the reaction solution, and reacted at 80°C for 4h. It was then removed under reduced pressure to obtain a purple crude product. The crude product was dissolved in 15 mL of acetonitrile, and 0.5 mL of triethylamine and 2-amino-5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com