Polypeptide crossing blood-brain barrier as well as derivative and application thereof
A blood-brain barrier and derivative technology, applied in the field of medical biology, can solve limited problems and achieve the effects of broad application prospects, enhanced targeting and therapeutic properties, and easy synthesis and modification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of polypeptide sequences across the blood-brain barrier
[0047] Synthesize the polypeptide sequence across the blood-brain barrier by artificial synthesis method,
[0048] The polypeptide sequence is as follows:
[0049] YLGASVPSPDPLEPT SEQ ID No. 1;
[0050] YLGASVPSPDPLEPTREQCELNPACDELSDQYGLKTAYKRIYGITI SEQ ID No. 2;
[0051] YLYQWLGAPVPYPDPLEPR SEQ ID No. 3;
[0052] YLYQWLGAPVPYPDPLEPREVCELNPDCDELADHIGFQEAYRRFYGPV SEQ ID No. 4.
[0053] The above-mentioned polypeptides are synthesized by conventional solid-phase synthesis or liquid-phase synthesis methods. Among them, the solid-phase synthetic peptide method is used to react from the amino acid at the C-terminal to the N-terminal. After the steps of resin activation, amino acid linkage, elution protection, and detection, the amino acid linkage is completed one by one, and then precipitated and centrifuged with excess ether, and the crude peptide is purified by HPLC. Afterwards, mass spec...
Embodiment 2
[0055] Example 2: Preparation of polypeptide probes across the blood-brain barrier
[0056] This embodiment provides a polypeptide probe, using the polypeptide prepared in Example 1, and combining it with ICG through an organic chemical reaction.
[0057] The specific method is purchase or synthesis. In this example, the probe is prepared by linking the polypeptide with ICG by click chemistry method, and the linker is the common DBCO. ICG with an activating functional group containing the amino group NH2 , carboxyl COOH, activated lipid NHS, maleimide MAL, mercapto SH, azide N 3 , alkyne ALK, and then coupled with the polypeptide prepared in Example 1 to obtain the blood-brain barrier polypeptide ICG probe.
Embodiment 3
[0058] Example 3: Detection of the ability of mice to cross the blood-brain barrier
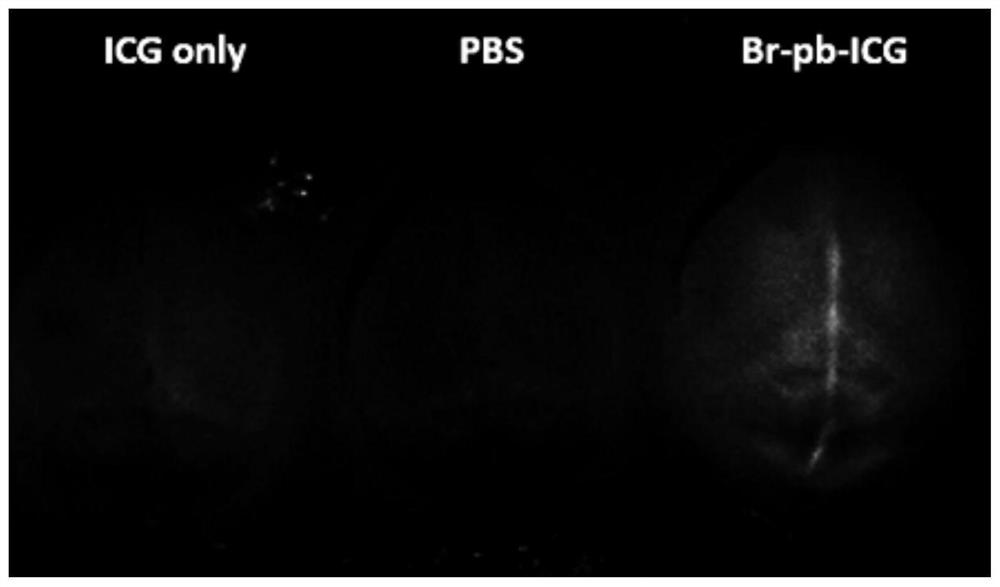
[0059] Adult mice were used, and the cross-blood-brain barrier polypeptide ICG probe (containing SEQ ID NO.1) prepared in Example 2, ICG solution (2mM) and PBS were injected into the tail vein, and each mouse was injected with 100 microliters. After 24 hours, Detection under small animal imager.
[0060] See the experimental results figure 1 , by comparing the results of the three groups, it can be seen that only the cross-blood-brain barrier polypeptide probe group has specific signals in the brain, while the other two groups do not show fluorescent signals in the brain. From the above experimental results, it can be seen that the blood-brain barrier-crossing polypeptide probe of the present invention can realize the effect of crossing the blood-brain barrier. Promote the transport efficiency across the blood-brain barrier, and then realize the transport of other active ingredients.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com