Application of Phthalazine Derivatives in Treating Hepatic Fibrosis
A technology for liver fibrosis and derivatives, which is applied in the field of phthalazine derivatives for the treatment of liver fibrosis diseases, can solve the problems that the targeted treatment of fibrosis diseases has not achieved satisfactory results, and achieve the effect of improving liver fibrosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of T-5-9:
[0041]
[0042]
[0043]
[0044] Wherein: Boc is tert-butoxycarbonyl, PyBOP is 1H-benzotriazol-1-yloxytripyrrolidinyl hexafluorophosphate; DIEA is N,N -Diisopropylethylamine; (Cl 3 CO) 2 CO is triphosgene.
[0045] 4-((4-((2-Fluorophenyl)carbamoyl)phenyl)carbamoyl)piperazine-1-carboxylate tert-butyl ester (2)
[0046] The 4-amino- N -(2-Fluorophenyl)benzamide (1) (4.75 g, 20.64 mmol) was dissolved in 30 mL of anhydrous dichloromethane, at room temperature, DIEA (7.19 mL, 41.28 mmol) and triphosgene (2.04 g, 6.88 mmol) in dichloromethane solution (20 mL), cooled to 0 °C and reacted for 3 hours, then added N -Boc-piperazine (5.77 g, 30.96 mmol), stirred at room temperature overnight, TLC detected that the raw material reacted completely, filtered, and the filtrate was concentrated to obtain a crude product, which was separated by column chromatography (eluent: dichloromethane: methanol=50:1 ) to obtain 7.12 g of a yellow solid with a...
Embodiment 2
[0055] Human semi-activated hepatic stellate cell line LX-2 cell assay
[0056] In this example, the LX-2 cell culture method: LX-2 cells were taken out from the -80 ℃ refrigerator, and the DMEM medium containing 10% fetal bovine serum was used in 10 cm 2 In a petri dish, 37 °C, 5% CO 2 Culture in the incubator, change the medium, passage, and take 3 to 6 passages for experiments.
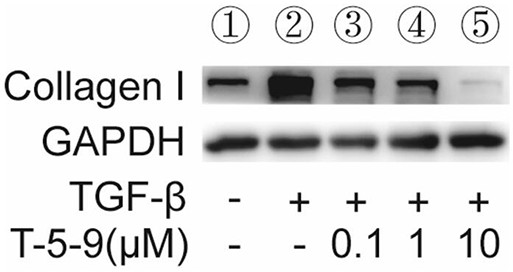
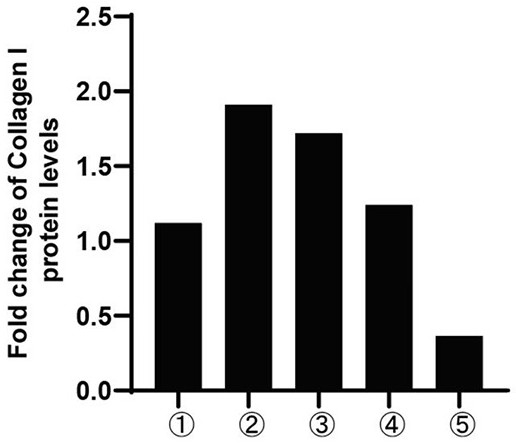
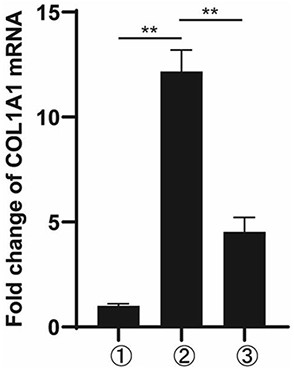
[0057] 1. Western Blot experiment
[0058] Human semi-activated hepatic stellate cells LX-2 (5*10 5 (1 / well), and set up ① blank control group, ② TGF-β stimulation group, ③ T-5-9 (0.1 μM) group, ④ T-5-9 (1 μM) group, and ⑤ T-5-9 (10 μM) group. When the cell density in the well was 70%, the cells were treated as shown in Table 1. After 24 h, the lysed protein was quantitatively collected, and Western blot was used to detect the expression of Collagen I protein in LX-2. The expression of Collagen I in the stimulation group and the drug-added group was tested to test whether T-5-9 could inhibit t...
Embodiment 3
[0078] Anti-hepatic fibrosis animal test
[0079] The modeling method of the mouse liver fibrosis disease model in this example: 24 male mice (6-week-old, weighing 18-22 grams) were randomly divided into 4 groups, with 6 mice in each group. By injecting 10% carbon tetrachloride (CCl 4 , 0.5 ml / 100 g body weight) induced liver fibrosis in wild-type mice for 8 weeks (three times a week). The mice in the control group were injected with the same amount of olive oil instead of carbon tetrachloride; the mice in the model group were injected with carbon tetrachloride; the mice in the positive drug group were injected with CCl 4 , and given colchicine (0.1 mg / kg, once a day, week 5-8); mice in the treatment group were injected with CCl intraperitoneally 4 , and given T-5-9 (12.5mg / kg, once a day, the 5th to 8th week); the follow-up experiments and the attached drawings were named as: ① control group, ② model group, ③ positive drug group, ④ T-5 -9 groups. Blood and liver samples f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com