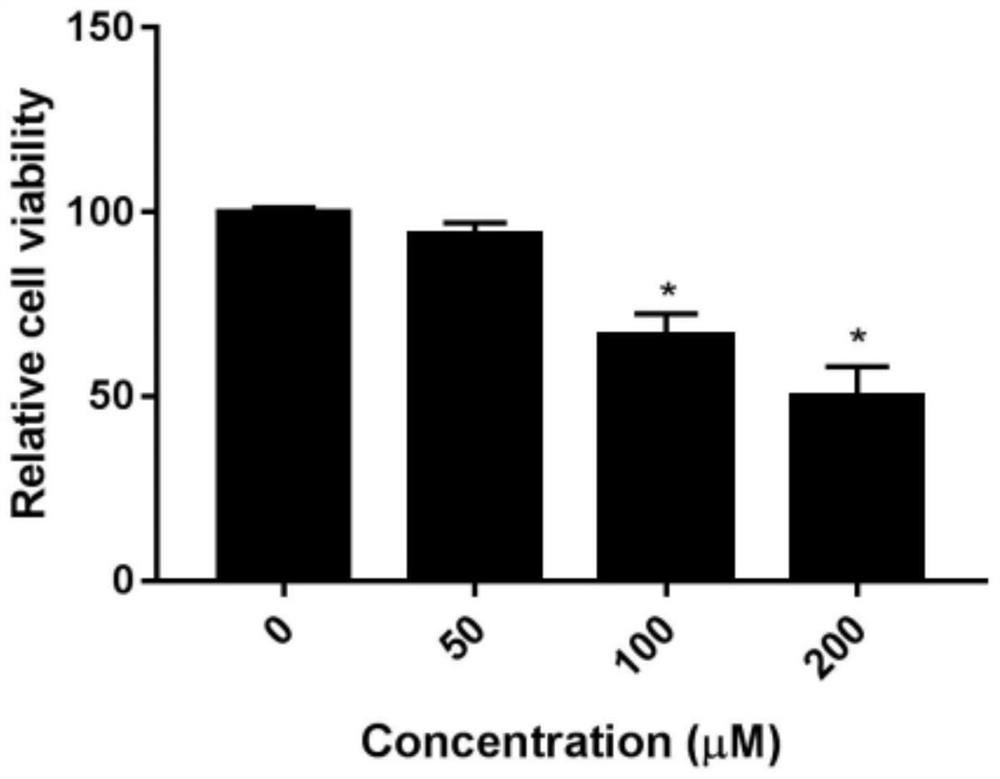
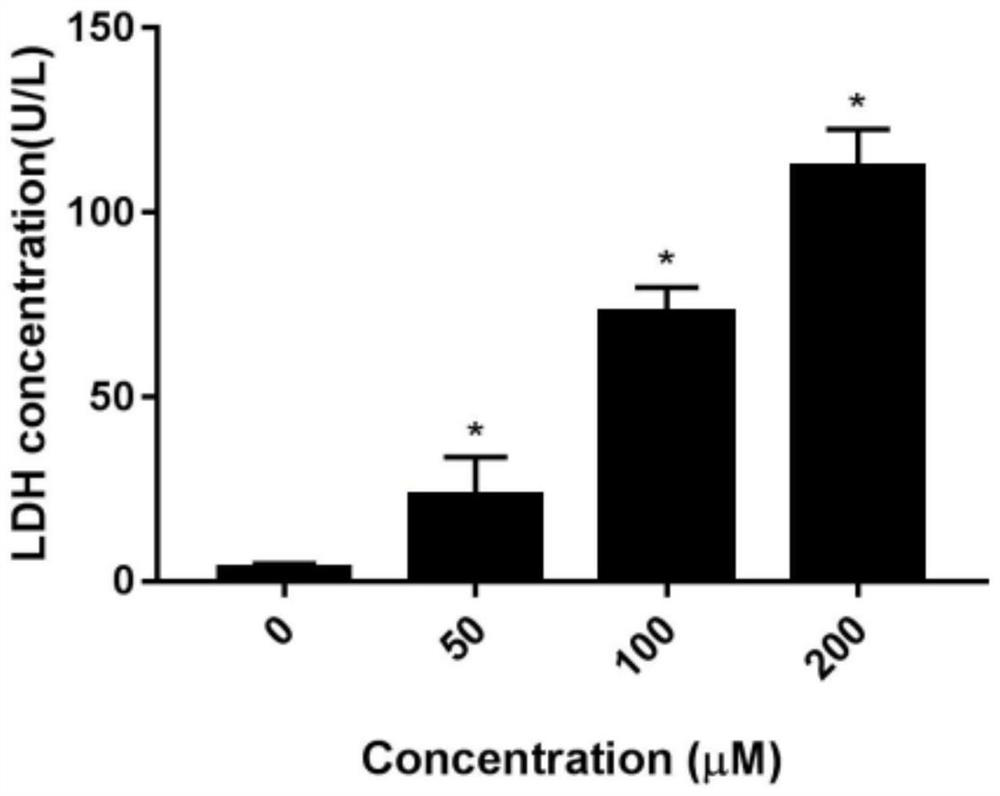
Human glomerular endothelial cell culture and drug detection toxicity evaluation method based on micro-fluidic chip
A microfluidic chip and endothelial cell technology, applied in artificial cell constructs, biochemical equipment and methods, microbial measurement/inspection, etc., can solve problems such as lack of models, poor predictive ability, and rising costs of developing new drugs. achieve precise results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] In this embodiment, the specific implementation steps are as follows
[0062] Step 1, chip pretreatment: suck out the moisturizing solution in the wells of the 1-6 columns of the liquid inlet holes and the 7th and 8th columns of the liquid drainage holes of the microfluidic cell chip, and pour PBS buffer into the wells 1-8, and then Aspirate and discard the PBS buffer in wells 1-8, add 325ul of PBS buffer into wells 1-6 again, seal and let stand at room temperature for 35min, and finally cover the microfluidic cell chip and the chip cover plate The microfluidic cell chip is rinsed and sterilized, and the gel pad is cleaned, sterilized, ultra-static and dried, and then placed on the microfluidic cell chip for standby;
[0063] Step 2, cell inoculation: empty the liquid in the No. 6 well of the microfluidic cell chip, and then add a concentration of 2x10 to the No. 6 well. 6 cells / ml of human glomerular endothelial cell solution 15ul, at the same time add 350ul of serum-...
Embodiment 2
[0074] In this embodiment, the difference from Embodiment 1 is that
[0075] Step 1: During chip and processing, add 300ul of PBS buffer solution into wells 1-6 again, seal and let stand at room temperature for 40min;
[0076] Step 2, during cell inoculation, add a concentration of 1x10 to the No. 6 well 6 cells / ml human glomerular endothelial cell solution 14ul;
[0077] Step A, in the test preparation, the test substance is centrifuged at 12000rpm for 8min, and the injection pressure of the No. 2 hole is set to 0.5psi;
[0078] Step B, activity assay, set pressure 3 psi, temperature 37 °C, in 5% CO 2 The conditions of gas concentration were incubated for 1 h.
Embodiment 3
[0080] In this embodiment, the difference from Embodiment 1 is that
[0081] Step 1: During chip and processing, add 350ul of PBS buffer solution into wells 1-6 again, seal and let stand at room temperature for 30min;
[0082] Step 2, during cell inoculation, add a concentration of 5x10 to the No. 6 well 6 cells / ml human glomerular endothelial cell solution 10ul;
[0083] Step A, in the test preparation, the test substance is centrifuged at 12000rpm for 10min, and the injection pressure of hole 2 is set to 1psi;
[0084] Step B, activity assay, set pressure 3 psi, temperature 37 °C, in 5% CO 2 The conditions of gas concentration were incubated for 4h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com