Ketorolac derivative as well as preparation method and application thereof
A technology of ketorolac and its derivatives, which is applied to the pharmaceutical composition containing the ketorolac derivatives, the field of ketorolac derivatives and its preparation, can solve the problems of short half-life and poor stability of ketorolac, and achieve The effect of high physical and chemical stability and good pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis of embodiment 1 compound N23
[0039]
[0040]At room temperature, weigh ketorolac (5.0g, 19.6mmol), add DMF (10mL) and stir to dissolve, then add 4-chloromethyl-5-methyl-1,3-dioxole-2- Ketone (2.91g, 19.6mmo), and then the reaction bottle was moved to an ice bath for stirring, and sodium carbonate (1.46g, 15.7mmol) was added to the reaction bottle in 5 batches, and the addition was completed. The reaction was moved to room temperature and the reaction was stirred overnight. After TLC detects that the reaction is complete, add ethyl acetate 100mL and water 70ml to the reaction bottle, shake and separate the liquids, and the organic layer is washed with 5% sodium thiosulfate (30ml), washed with water (30ml), and dried over anhydrous sodium sulfate. , concentrated, made sand, and obtained 6.5 g of white solid by flash column chromatography, yield 90.4%.
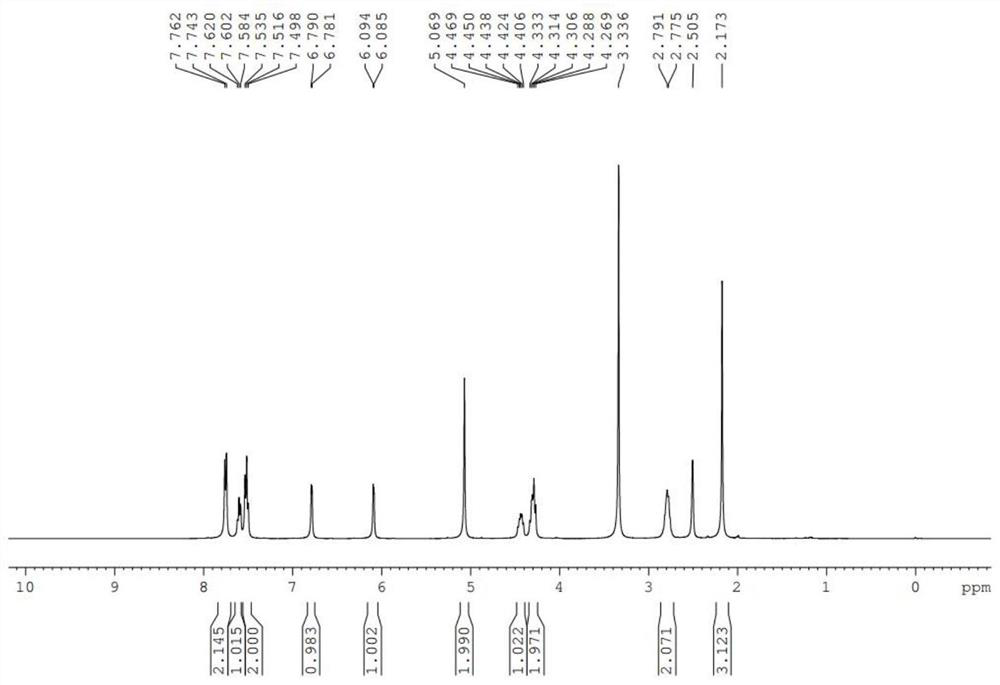
[0041] 1 H NMR (400MHz, CDCl 3 )δ7.762-7.743(m,2H),7.620-7.584(m,1H),7.535-7.498(m.2H),6.7854(d,J...
Embodiment 2
[0043] Embodiment 2: the synthesis of compound N23 (S)
[0044]
[0045] The experimental method is the same as in Example 1, except that ketorolac is replaced by S-ketorolac.
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.762-7.743(m,2H),7.620-7.584(m,1H),7.535-7.498(m.2H),6.7854(d,J=3.6Hz,1H),6.090(d,J=3.6Hz ,1H),4.920(s,2H),4.612-4.546(m,1H),4.483-4.416(m,1H),4.142-4.089(m,1H),2.973-2.776(m,2H),2.1769(s ,3H)
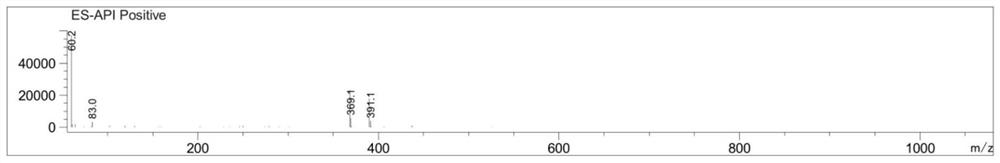
[0047] ESI-MS m / z=390.1,[M+Na] + .
Embodiment 3
[0048] The synthesis of embodiment 3 compound N23
[0049] At room temperature, weigh ketorolac (5.0g, 19.6mmol), add THF (10mL) and stir to dissolve, then add 4-chloromethyl-5-methyl-1,3-dioxole-2- Ketone (2.91g, 19.6mmol), and then the reaction flask was moved to an ice bath for stirring, and sodium carbonate (1.46g, 15.7mmol) was added to the reaction flask in 5 batches, and the addition was completed. The reaction was moved to room temperature and the reaction was stirred overnight. After TLC detects that the reaction is complete, add ethyl acetate 100mL and water 70ml to the reaction bottle, shake and separate the liquids, and the organic layer is washed with 5% sodium thiosulfate (30ml), washed with water (30ml), and dried over anhydrous sodium sulfate. , concentrated, made sand, and obtained 6.2 g of a white solid by flash column chromatography, with a yield of 86.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com