Constrained conditionally activated binding proteins
A constrained, fusion protein technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
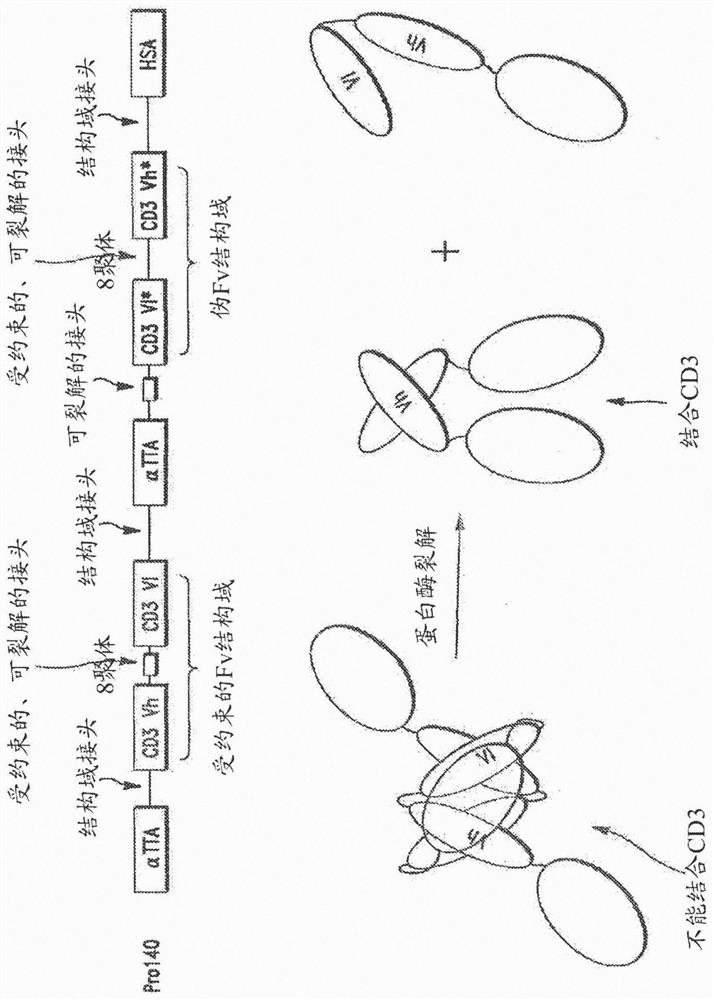
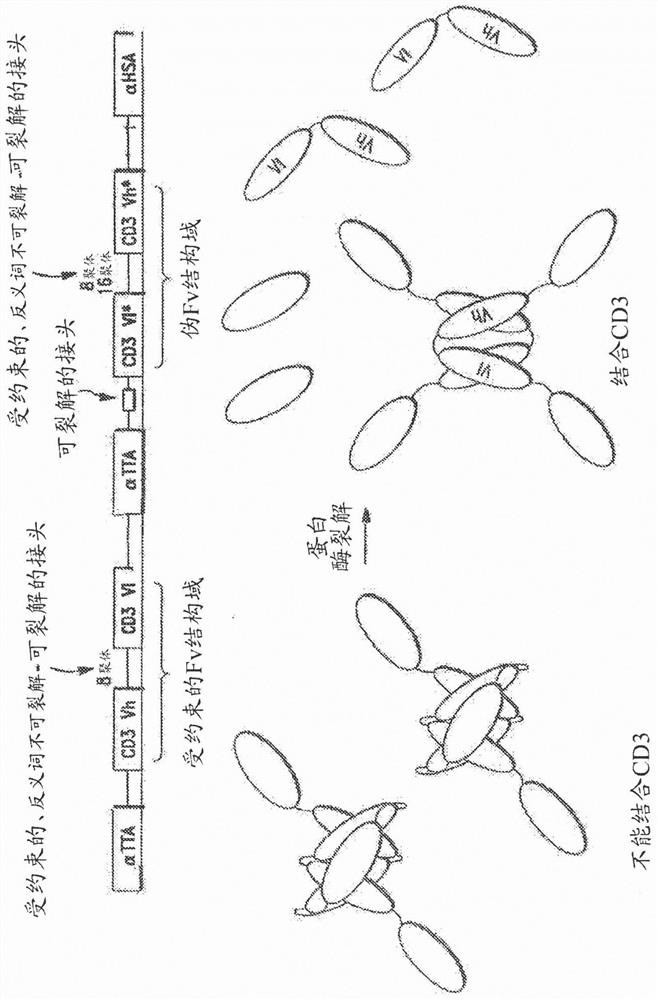
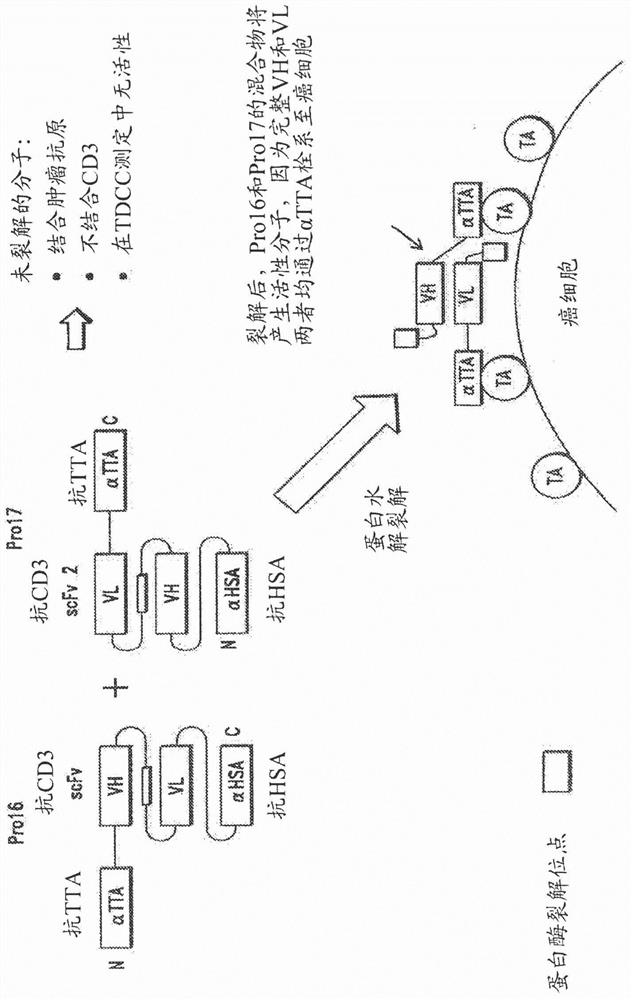
[0225] I. fusion protein of the present invention
[0226] Fusion proteins of the invention have many different components (generally referred to herein as domains) linked together in a variety of ways. Some of the domains are binding domains that each bind to a target antigen (eg, TTA or CD3). Because they bind to more than one antigen, they are referred to herein as "multispecific"; for example, the prodrug constructs of the invention can bind to TTA and CD3, and are therefore "bispecific". Proteins can also have higher specificity; for example, if the first αTTA binds to EGFR, the second αTTA binds to EpCAM, and an anti-CD3 binding domain is present, this would be a "trispecific" molecule. Similarly, adding an anti-HSA binding domain to this construct would be "tetraspecific", as in Figure 3B shown in .
[0227] As will be appreciated by those skilled in the art, proteins of the invention may have different valences and be multispecific. That is, proteins of the invent...
Embodiment 1
[0438] A. Example 1: Construction and purification of Pro constructs
[0439] transfection
[0440] Each protein (eg, single proteins of formats 1, 2 and 4) or paired constructs (format 3) was expressed from individual expression vectors (pcDNA3.4 derivatives). Equal amounts of plasmid DNA encoding a pair of half-Cobra or single-stranded constructs were mixed and transfected into Expi293 cells following the manufacturer's transfection protocol. Five days after transfection, conditioned medium was harvested by centrifugation (6000rpm x 25') and filtration (0.2uM filter). Protein expression was confirmed by SDS-PAGE. The construct was purified and the final buffer composition was: 25 mM citrate, 75 mM arginine, 75 mM NaCl, 4% sucrose, pH 7. The final formulation was stored at -80°C.
[0441] Activation of MMP9
[0442] Recombinant human (rh)MMP9 was activated according to the following protocol. Recombinant human MMP-9 (R&D #911-MP-010) is 0.44mg / ml (4.7uM). Phenylmer...
Embodiment 2
[0450] B. Example 2: T cell-dependent cell cytotoxicity (TDCC) assay
[0451] Firefly luciferase-transduced HT-29 cells were grown to approximately 80% confluency and detached with Versene (0.48 mM EDTA-Ca-Mg in PBS). Cells were centrifuged and resuspended in TDCC medium (5% heat-inactivated FBS in RPMI 1640 with HEPES, GlutaMax, sodium pyruvate, non-essential amino acids and β-mercaptoethanol). Purified human P pan-T cells were thawed, centrifuged and resuspended in TDCC medium.
[0452] A co-culture of HT-29_Luc cells and T cells was added to a 384-well cell culture plate. Serial dilutions of COBRA were then added to the co-culture and incubated at 37 °C for 48 h. Finally, an equal volume of SteadyGlo Luciferase Assay Reagent was added to the plate and incubated for 20 minutes. Plates were read on a Perkin Elmer Envision with an exposure time of 0.1 s / well. Total fluorescence was read and data analyzed on GraphPad Prism 7 or version 8.3.1 (depending on timing).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com