SiRNA for specifically knocking down TNF-alpha gene expression and application thereof
An α gene and species-specific technology, applied in the field of siRNA, to improve the ability to resist enzymolysis and increase the residence time in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
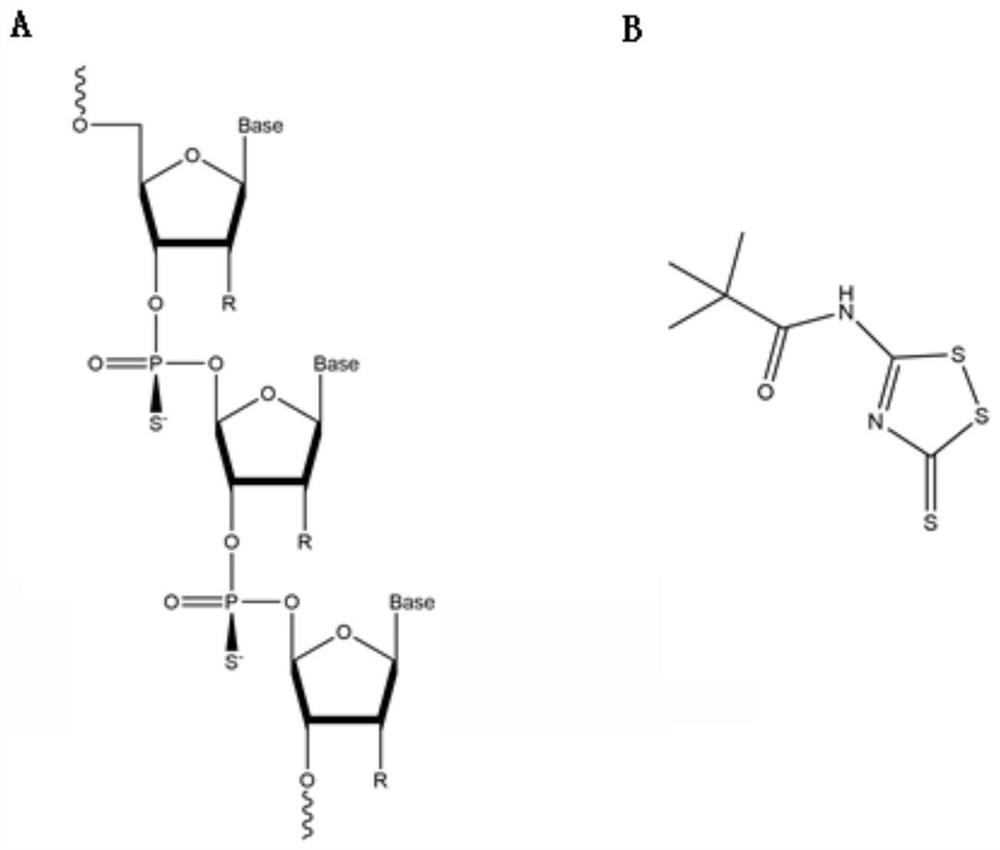
[0029] Example 1: Obtaining unmodified or modified siTNF-α
[0030] (1) The siRNA sequence is designed online in 'siRNA Target Finder', and the 5' untranslated region (5'UTR) and 3' untranslated region (3'UTR) and the sequence near the start codon should not be used as the design when designing The template of siRNA should also avoid the junction region between exon and exon. Search for the ideal siRNA starting from the 100bp nucleotides downstream of the start codon AUG of the target gene. Three pairs of siTNF-α were designed. One pair of siRNAs with the highest level of specific knockdown was confirmed by preliminary experiments (the nucleotide sequences of the sense strand and the antisense strand are shown in SEQ ID NO.2 and SEQ ID NO.3, respectively).
[0031](2) Using siRNAs with nucleotide sequences as shown in SEQ ID NO.4 and SEQ ID NO.5 respectively as negative controls, the siRNA with the highest specific knockdown level and the negative control were modified diffe...
Embodiment 2
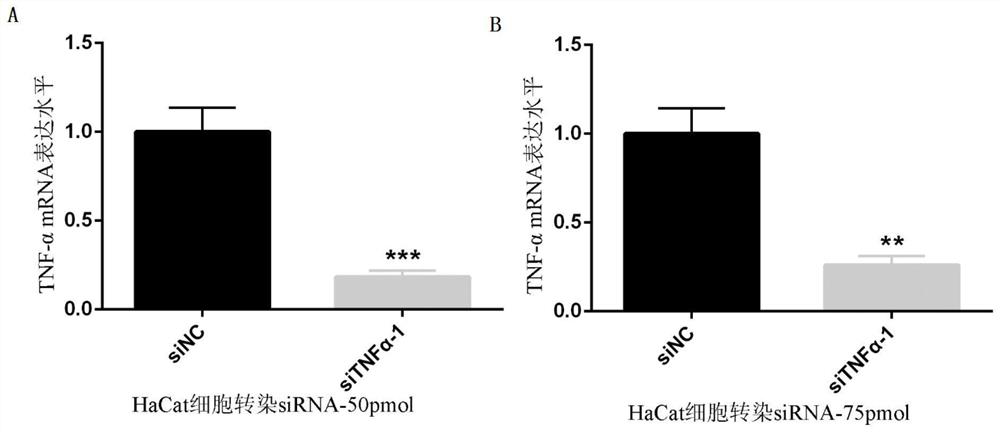
[0042] Example 2: Unmodified or modified siTNF-α transfected cells, detecting the mRNA expression level of TNF-α in cells
[0043] (1) Transfection
[0044] ①To quantify siRNA, dilute to 20uM with DEPC water.
[0045] ②HaCat cells were passaged to 12-well plates, and the density reached 30%-50% the next day before transfection.
[0046] ③For transfection, add 2.5ul siRNA to 50ul DMEM medium as tube A, add 5ul lip3000 to 50ul DMEM medium as tube B, add tube A to tube B, mix well, and let stand at room temperature for 15 minutes. Add dropwise to a 12-well plate. Set up control NC group, unmodified or modified siTNF-α group, and do 3 parallels for each experimental group.
[0047] (2) RNA extraction
[0048] Use the Tiangen kit 'RNA prep Pure Cell / Bacteria Kit', the article number is DP430, the specific operation is as follows:
[0049] ① Remove the cell culture medium, add 500ul PBS to each well to wash the cells, discard, twice.
[0050] ②Add 350ul lysate RL to each well,...
Embodiment 3
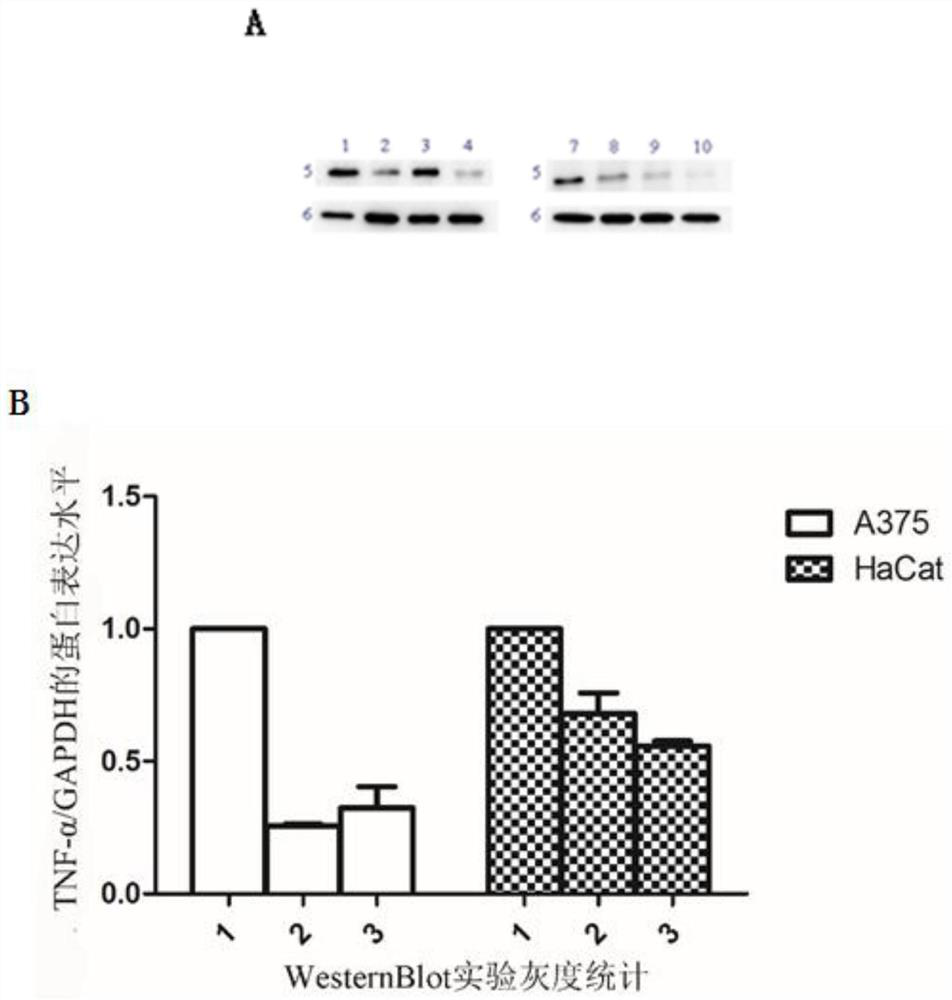
[0072] Example 3: Unmodified or modified siTNF-α transfected cells, detecting the protein expression level of TNF-α in cells
[0073] (1) Transfection
[0074] ①To quantify siRNA, dilute to 20uM with DEPC water. Confirm that the transfection concentration is 50 pmol.
[0075] ②HaCat cells were passaged to 12-well plates, and the density reached 30%-50% the next day before transfection.
[0076] ③For transfection, add 2.5ul siRNA to 50ul DMEM medium as tube A, add 5ul lip3000 to 50ul DMEM medium as tube B, add tube A to tube B, mix well, and let stand at room temperature for 15 minutes. Add dropwise to a 12-well plate. Set up control NC group, unmodified or modified siTNF-α group, and do 3 parallels for each experimental group.
[0077] ④ One additional well was set up, and 50 pmol of modified siTNF-α with FAM fluorescent group was transfected.
[0078] (2) After the cells were cultured for 48 hours, the cells were collected and the protein was extracted
[0079] ① After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com