Preparation method and application of realgar nanoparticles
A nanoparticle, realgar technology, applied in nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of limited clinical application, low bioavailability, insoluble in water, etc., to prolong the residence time in the body and reduce toxic and side effects. , the effect of maximizing the curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
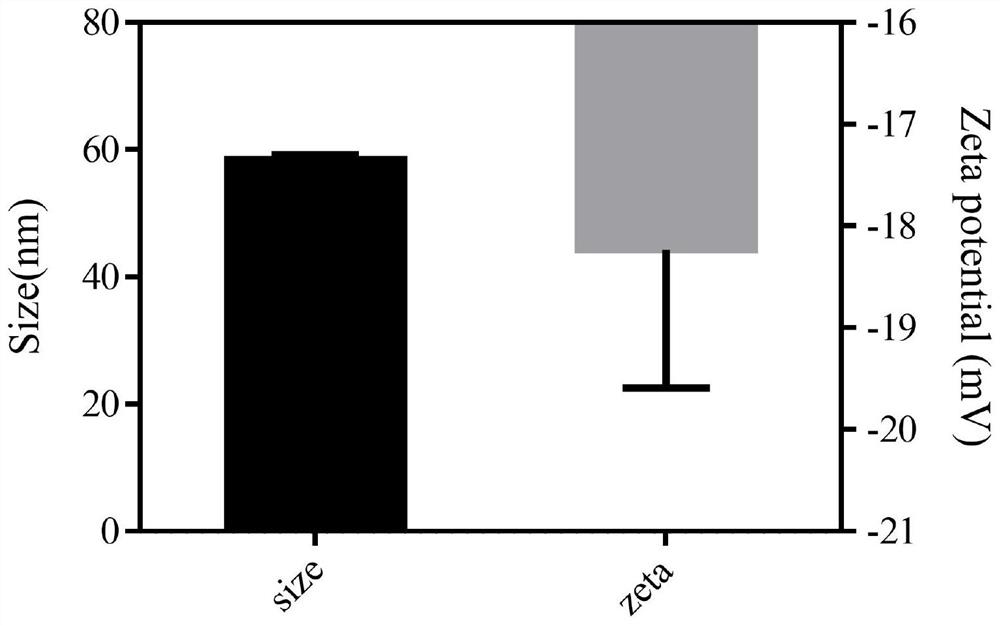
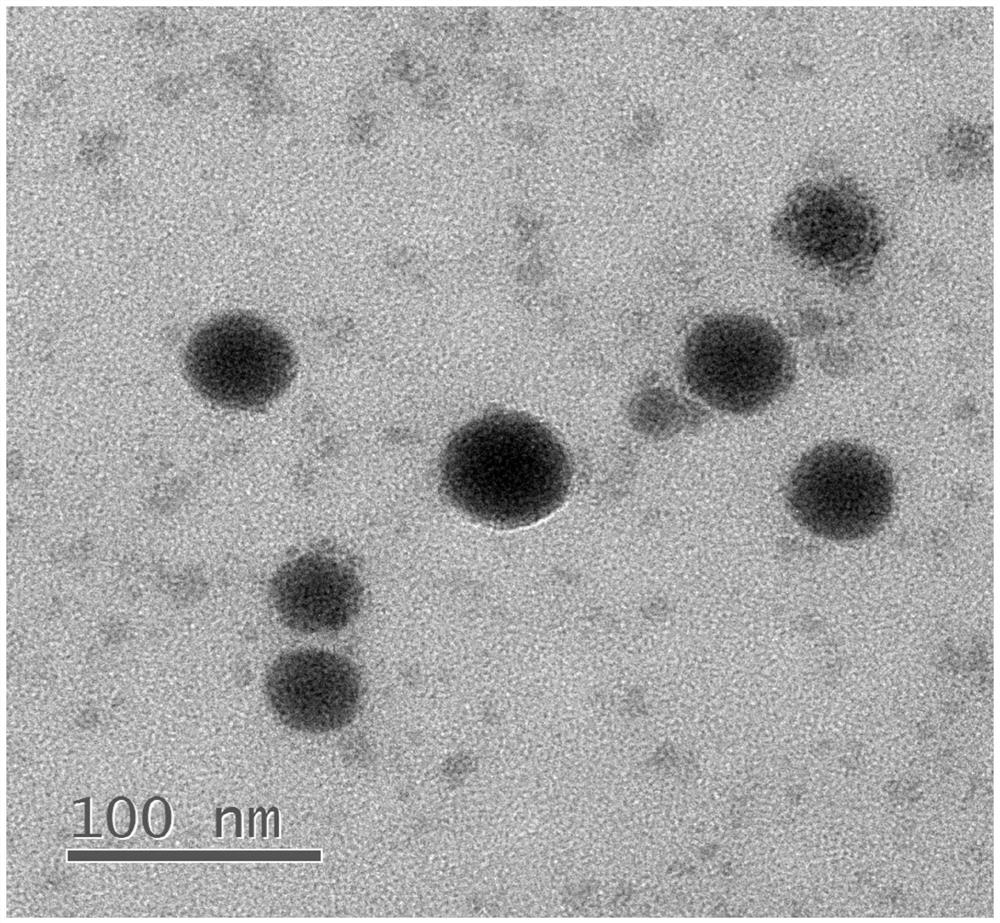
[0039] A realgar nanoparticle, comprising realgar and bovine serum albumin.
[0040] The nano-realgar compound drug of the present embodiment is prepared by the following method:
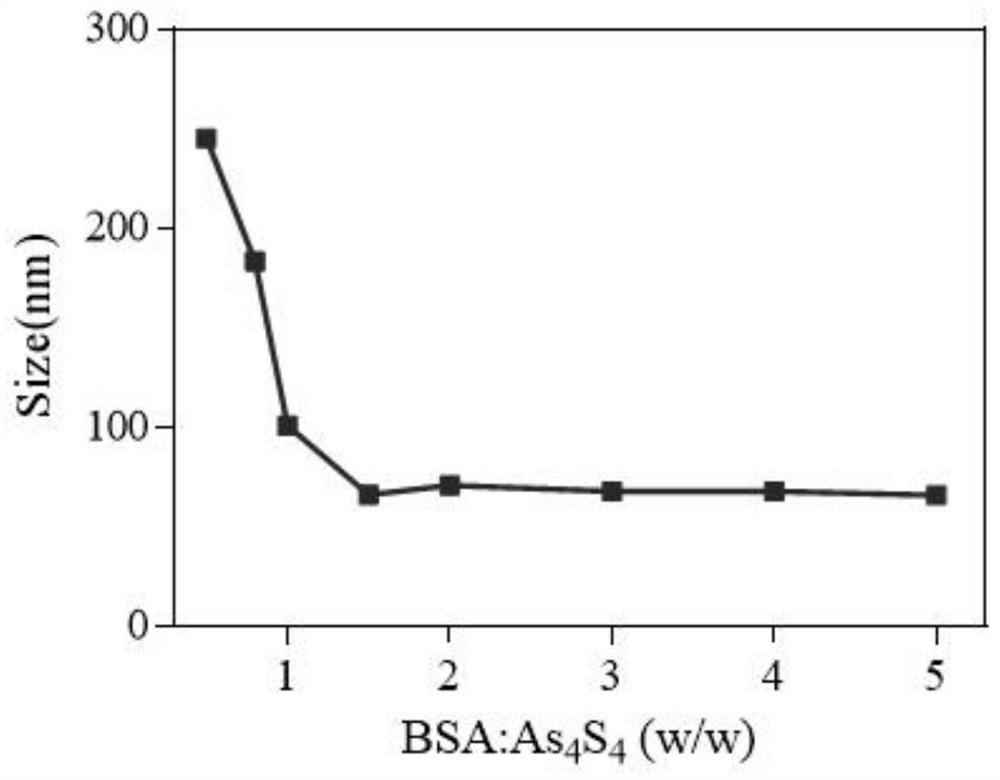
[0041] Will As 4 S 4 Dissolve it in ethylenediamine at a concentration of 20 mg / mL, sonicate for 15 minutes, centrifuge at 15,000 rpm for 5 minutes, and take the supernatant. 2mLAs 4 S 4 Disperse the supernatant in 8mL of water, add 3ml of 20mg / ml BSA solution, stir for 5min, add 7mL of 4MHCl, stir for 30min, and dialyze in water for 8h with a dialysis bag with MWCO=3500Da to obtain realgar nanoparticles: As 4 S 4 @BSA NPs.
Embodiment 2
[0043] A kind of realgar nanoparticles, including realgar, bovine serum albumin and folic acid.
[0044] The realgar nanoparticles of the present embodiment are prepared by the following method:
[0045] (1) According to the molar ratio of FA, EDC, and NHS: 1:2:1, it was dissolved in DMSO and stirred for 30 minutes in the dark to obtain FA with a concentration of 25 mg / ml. Slowly add the FA solution into the BSA carbonate buffer solution dropwise, stir at room temperature for 24 hours, dialyze in PBS (10mm, pH=7.4) with a dialysis bag of MWCO=3500Da for 2 days, dialyze in water for 1 day, collect and lyophilize to obtain BSA -FA.
[0046] (2) As 4 S 4 Dissolve it in ethylenediamine at a concentration of 20 mg / mL, sonicate for 15 minutes, centrifuge at 15,000 rpm for 5 minutes, and take the supernatant. At the same time, 60 mg of lyophilized BSA-FA was dissolved in 3 ml of ultrapure water, and then 2 mL of As 4 S 4 Disperse the supernatant in 8mL of water, slowly add BSA-...
Embodiment 1 and Embodiment 2
[0054] Example 1 and Example 2 The cytotoxicity of realgar nanoparticles to human chronic myelogenous leukemia cell K562:
[0055] (1) The logarithmic growth K562 cells were taken, diluted with IMDM medium containing 10% fetal bovine serum to a cell suspension with a density of 50000 cells / mL, and inoculated into a 96-well culture plate at 100 μL per well. In a carbon dioxide incubator (37°C, 5% CO 2 , saturated humidity) after culturing for 2 h, the culture medium was discarded by centrifugation.
[0056] (2) Add 100 μL of As diluted to different concentrations with culture medium to each well 4 S 4 @BSA-FA, As 4 S 4 @BSA (concentration in As 4 S 4 Concentration meter, respectively 0, 0.5, 1, 5, 10, 20, 50μg / ml), the same concentration was repeated for 6 replicate wells, and incubated for 48h.
[0057] (3) Centrifuge to remove the medium, add 100 μL of MTT solution (0.5 mg / mL, diluted in IMDM) to each well, continue to incubate for 4 hours, terminate the culture, and d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com